Synthesis method of indoxacarb intermediate semicarbazone
A technology of semicarbazone and synthetic method, which is applied in the field of synthesis of indoxacarb intermediate semicarbazone, can solve the problems of reduced yield, carbon-nitrogen double bond breakage, high toxicity, etc., and avoid high cost, Good safety, economy and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
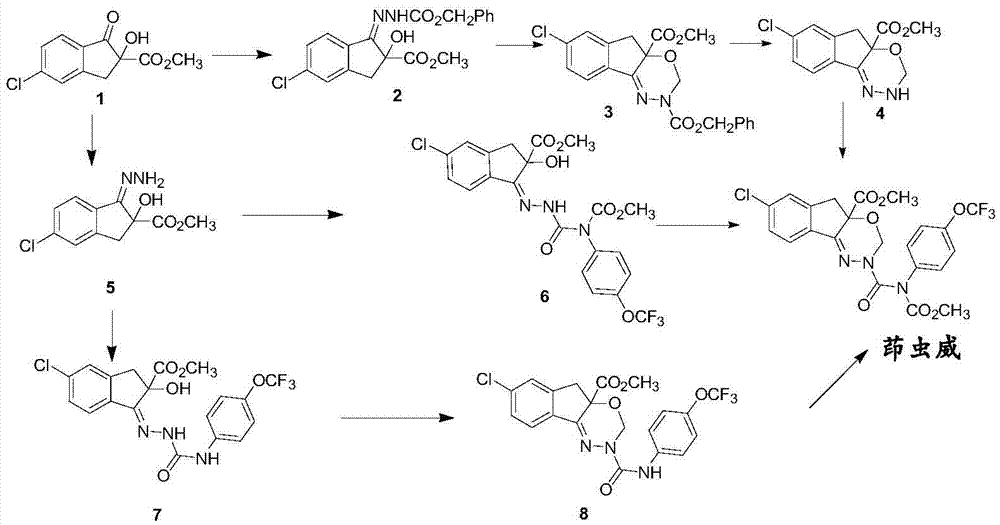
[0030] A kind of synthetic method of indoxacarb intermediate semicarbazone, concrete steps are as follows:
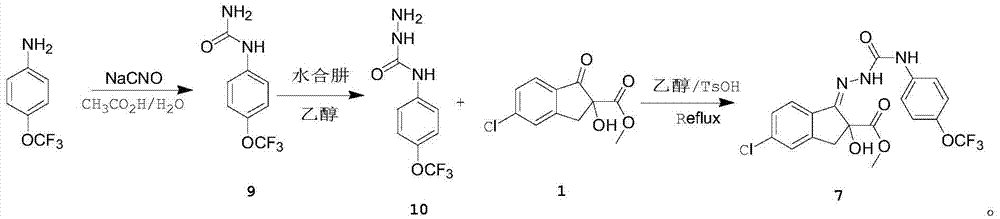
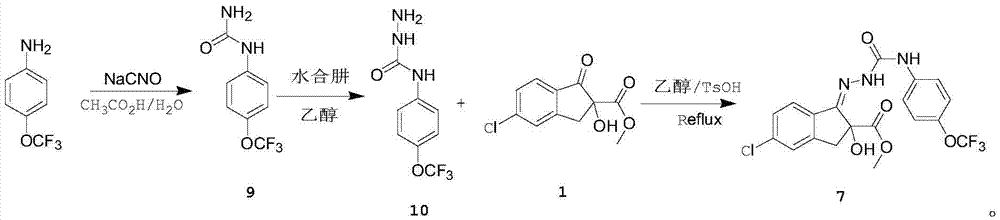
[0031] (1), add 17.7g (0.1mol) 4-trifluoromethoxyaniline, 50mL acetic acid and 100mL water successively in 500mL four-neck flask under nitrogen protection; 14.4g (0.22mol) mass fraction is 90% Dissolve sodium isocyanate in 40mL of acetic acid and 100mL of water, add the sodium isocyanate solution dropwise to the 4-trifluoromethoxyaniline solution in an ice-water bath, raise the temperature to 20°C, stir and react for 12 hours, and analyze in the central control The conversion rate of 4-trifluoromethoxyaniline was 97%, then cooled to 0-5°C and kept for 2 hours, and suction filtered to obtain off-white solid 4-trifluoromethoxyphenylurea with a yield of 85%;
[0032] (2), in a 250ml four-necked flask equipped with a condenser, add 10.5g (48mmol) of p-trifluoromethoxyphenyl urea obtained in step 1, 5.7g (144mmol) of hydrazine hydrate with a mass fraction of 80% , 50mL of e...
Embodiment 2
[0035] A kind of synthetic method of indoxacarb intermediate semicarbazone, concrete steps are as follows:
[0036](1), under nitrogen protection, add 34g (0.2mol) 4-trifluoromethoxyaniline, 100mL acetic acid and 200mL water successively in 1L four-neck flask; Dissolve potassium cyanate in 80mL acetic acid and 200mL water, add the potassium isocyanate solution dropwise to the 4-trifluoromethoxyaniline solution in an ice-water bath, heat up to 30°C and stir for 24 hours. The central control analysis 4- The conversion rate of trifluoromethoxyaniline was 99%, and then cooled to 0-5° C. for 1 hour, and suction filtered to obtain off-white solid 4-trifluoromethoxyphenylurea, with a yield of 85%;
[0037] (2), in a 250ml four-necked flask equipped with a condenser, add 10.5g (48mmol) of p-trifluoromethoxyphenyl urea obtained in step 1, 5.7g (144mmol) of hydrazine hydrate with a mass fraction of 80% , 50mL of ethanol, and then heated up to 80°C for reflux, reflux for 24 hours, and t...
Embodiment 3
[0040] A kind of synthetic method of indoxacarb intermediate semicarbazone, concrete steps are as follows:
[0041] (1), add 17.7g (0.1mol) 4-trifluoromethoxyaniline, 50mL acetic acid and 100mL water successively in 500mL four-neck flask under nitrogen protection; 14.4g (0.22mol) mass fraction is 90% Dissolve sodium isocyanate in 40mL of acetic acid and 100mL of water, add the sodium isocyanate solution dropwise to the 4-trifluoromethoxyaniline solution in an ice-water bath, heat up to 25°C and stir for 12 hours. - The conversion rate of trifluoromethoxyaniline is 95%, then cooled to 0-5°C and kept for 2 hours, and suction filtered to obtain off-white solid 4-trifluoromethoxyphenylurea, with a yield of 85%;
[0042] (2) In a 250ml four-necked flask equipped with a condenser, add 10.5g (48mmol) of p-trifluoromethoxyphenyl urea obtained in step 1 and 5.7g (144mol) of hydrazine hydrate with a mass fraction of 80%. , 50mL of ethanol, then heated up to 80°C for reflux, reflux for ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com