A kind of hydroxypyridone ligand and its application
A hydroxypyridone and ligand technology, applied in the directions of organic chemistry, antidote, drug combination, etc., can solve the problems of high synthesis cost, difficult to remove nuclide, large side effects, etc., and achieves safe synthesis route, easy complexation, cost low effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
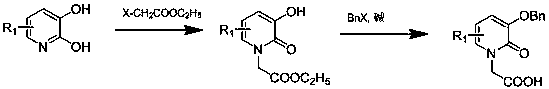
[0049] Example 1 Synthesis of 1,3-bis[(3-hydroxy-2-pyridone)-1-ethyl]acetamido-propanediamine (3,2-HOPO-3C) The synthetic route of this example is as follows :
[0050]
[0051] Specifically include the following steps:
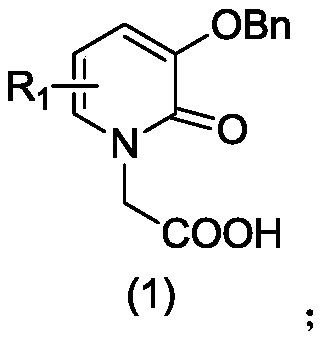
[0052] Weigh 11.1 g of 2,3-dihydroxypyridine ((1), 0.1 mol) in the reaction flask, add 83.5 g of ethyl bromoacetate (0.5 mol), and mix N 2 Pass to the reaction liquid below the surface and stir for 1h, then under N 2Protection, reflux reaction at 150°C for 24h. After the reaction, the reaction bottle was removed from the oil bath, cooled to room temperature, and a solid was precipitated, then the reaction solution was filtered, the solid was washed with acetone for 3-5 times, and then recrystallized with ethanol. After drying in a vacuum oven for 24 hours, the off-white product (2) was obtained, with a yield of 13.7 g and a yield of 70%. Its NMR and mass spectrometry test results are as follows:
[0053] 1 H NMR (400MHz, DMSO) δ7.43-7.36(m,2H),7.34-7...
Embodiment 2
[0059] Example 2 Synthesis of 2,2'-oxybis[(3-hydroxyl-2-pyridone)-1-ethyl]acetamido-ethylamine (3,2-HOPO-2NO)
[0060] The synthetic route of the present embodiment is as follows:
[0061]
[0062] Specifically include the following steps:
[0063] Weigh 11.1 g of 2,3-hydroxypyridine ((1), 0.1 mol) in the reaction flask, add 83.5 g of ethyl bromoacetate (0.5 mol), and mix N 2 Pass to the reaction liquid below the surface and stir for 1h, then under N 2 Protection, reflux reaction at 150°C for 24h. After the reaction, the reaction bottle was removed from the oil bath, cooled to room temperature, and a solid was precipitated, then the reaction solution was filtered, the solid was washed with acetone for 3-5 times, and then recrystallized with ethanol. After drying in a vacuum oven for 24 hours, the off-white product (2) was obtained, with a yield of 13.7 g and a yield of 70%.
[0064] Get the above-mentioned product (2) 10g (0.05mol) and dissolve in 300ml of 90% methanol ...
Embodiment 3
[0069] Example 3 Synthesis of 3,3'-oxybis[(3-hydroxyl-2-pyridone)-1-ethyl]acetamido-propylamine (3,2-HOPO-3NO)
[0070] The synthetic route of the present embodiment is as follows:
[0071]
[0072] Specifically include the following steps:
[0073] Weigh 11.1 g of 2,3-hydroxypyridine ((1), 0.1 mol) in the reaction flask, add 83.5 g of ethyl bromoacetate (0.5 mol), and mix N 2 Pass to the reaction liquid below the surface and stir for 1h, then under N 2 Protection, reflux reaction at 150°C for 24h. After the reaction, the reaction bottle was removed from the oil bath, cooled to room temperature, and a solid was precipitated, then the reaction solution was filtered, the solid was washed with acetone for 3-5 times, and then recrystallized with ethanol. After drying in a vacuum oven for 24 hours, the off-white product (2) was obtained, with a yield of 13.7 g and a yield of 70%.
[0074] Get the above-mentioned product (2) 10g (0.05mol) and dissolve in 300ml of 90% methanol...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com