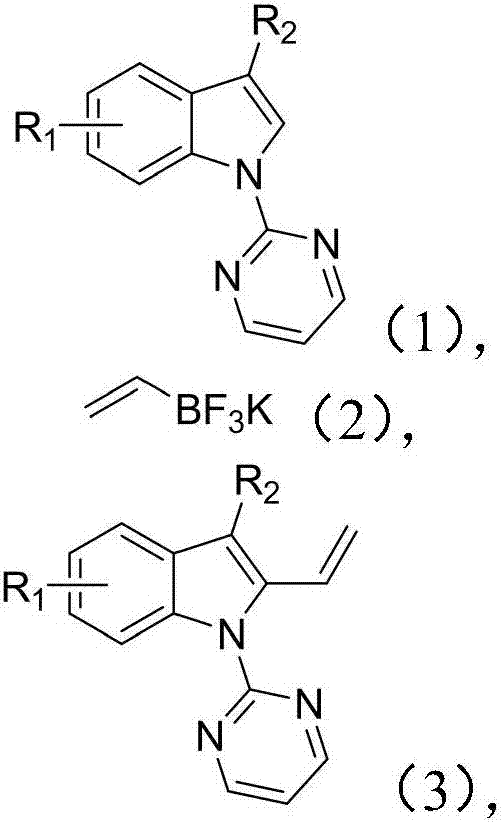
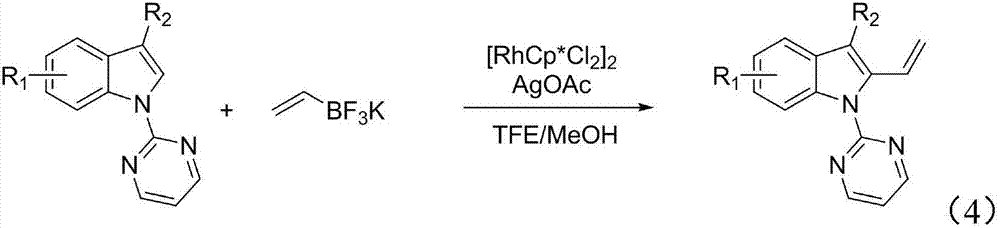
Method of synthesizing 2-vinyl indole derivative
A technology of ethylene indole derivatives and ethylene, which is applied in the field of synthesizing 2-vinylindole derivatives, can solve the problems of unstable yield, inability to synthesize 2-vinylindole derivatives, large limitations, etc., and achieve reaction Simple steps, easy operation, and highly responsive effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036]
[0037] In this example: R 1 is hydrogen; R 2 For methyl.
[0038] Step: In a 10mL reaction flask, add compound I-1 (0.2mmol), potassium vinyl trifluoroborate (0.3mmol), AgOAc (0.3mmol), [RhCp*Cl 2 ] 2(2mol%), methanol (0.5mL), trifluoroethanol (0.5mL), the mixture was reacted at 40°C, and the reaction was detected by TLC (thin layer chromatography) until the reaction was complete. Post-processing purification: cooling to room temperature, concentrated under pressure. The crude product was separated and purified by silica gel column chromatography, and the mobile phase was a mixed solvent of V (petroleum ether): V (ethyl acetate) = 5:1 to obtain a pure product, a white solid, with a yield of 82%.
[0039] Compound II-1 was tested:
[0040] The melting point is 99-100°C;
[0041] 1 H NMR (400MHz, CDCl 3 )δ8.77(d, J=4.8Hz, 2H), 8.27(d, J=7.9Hz, 1H), 7.57(d, J=7.2Hz, 1H), 7.31-7.21(m, 2H), 7.11- 7.02(m,2H),5.50-5.38(m,2H),2.44(s,3H).
[0042] 13 C NMR (100MH...
Embodiment 2
[0045]
[0046] In this example: R 1 is methyl; R 2 For methyl.
[0047] Step: In a 10mL reaction flask, add compound I-2 (0.2mmol), potassium vinyltrifluoroborate (0.3mmol), AgOAc (0.3mmol), [RhCp*Cl 2 ] 2 (2mol%), methanol (0.5mL), trifluoroethanol (0.5mL), the mixture was placed at 40°C for reaction, and TLC (thin layer chromatography) detected that the reaction was complete. Post-processing purification: cooling to room temperature, concentrated under pressure. The crude product was separated and purified by silica gel column chromatography, and the mobile phase was a mixed solvent of V (petroleum ether): V (ethyl acetate) = 5:1 to obtain a pure product, a white solid, with a yield of 72%.
[0048] Compound II-2 was tested:
[0049] The melting point is 116-117°C;
[0050] 1 H NMR (400MHz, CDCl 3 )δ8.77(d,J=4.8Hz,2H),8.02(d,J=8.4Hz,1H),7.13–7.09(m,2H),6.97–6.90(m,2H),5.45(dd,J =11.5,1.6Hz,1H),5.29(dd,J=17.7,1.6Hz,1H),2.76(s,3H),2.62(s,3H).
[0051] 13 C NMR (...
Embodiment 3
[0054]
[0055] In this example: R 1 is benzyloxy; R 2 methyl.
[0056] Step: In a 10mL reaction flask, add compound I-3 (0.2mmol), potassium vinyltrifluoroborate (0.3mmol), AgOAc (0.3mmol), [RhCp*Cl 2 ] 2 (2mol%), methanol (0.5mL), trifluoroethanol (0.5mL), the mixture was placed at 40°C for reaction, and TLC (thin layer chromatography) detected that the reaction was complete. Post-processing purification: cooling to room temperature, concentrated under pressure. The crude product was separated and purified by silica gel column chromatography, and the mobile phase was a mixed solvent of V (petroleum ether): V (ethyl acetate) = 5:1 to obtain a pure product, a white solid, with a yield of 61%.
[0057] Compound II-3 was tested:
[0058] The melting point is 107-108°C;
[0059] 1 H NMR (400MHz, CDCl 3 )δ8.77(d, J=4.8Hz, 2H), 7.80(d, J=8.4Hz, 1H), 7.51(d, J=7.3Hz, 2H), 7.40(t, J=7.4Hz, 2H) ,7.34–7.31(m,1H),7.17–7.08(m,2H),6.93(dd,J=17.7,11.5Hz,1H),6.68(d,J=7.9Hz,1H),5...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com