A kind of no donor type statin derivative, preparation method and application
A statin and reaction technology, applied in the field of N-aryl-N'-hydroxyguanidine NO-donating statin derivatives and their preparation, to treat peripheral ischemia, improve NO release activity, lower cholesterol and triglycerides horizontal effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
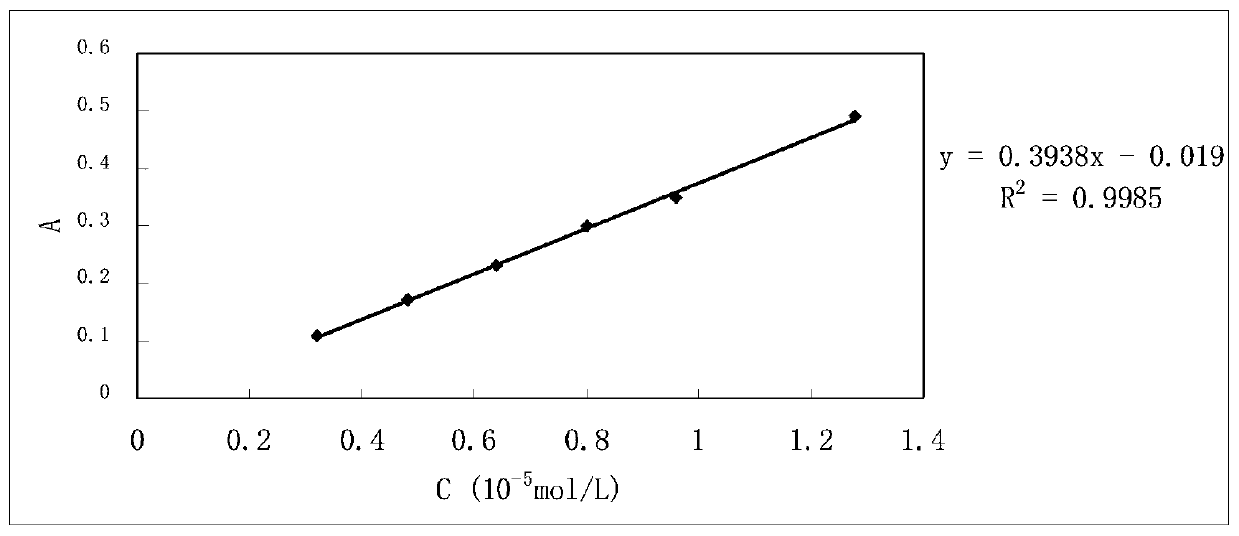
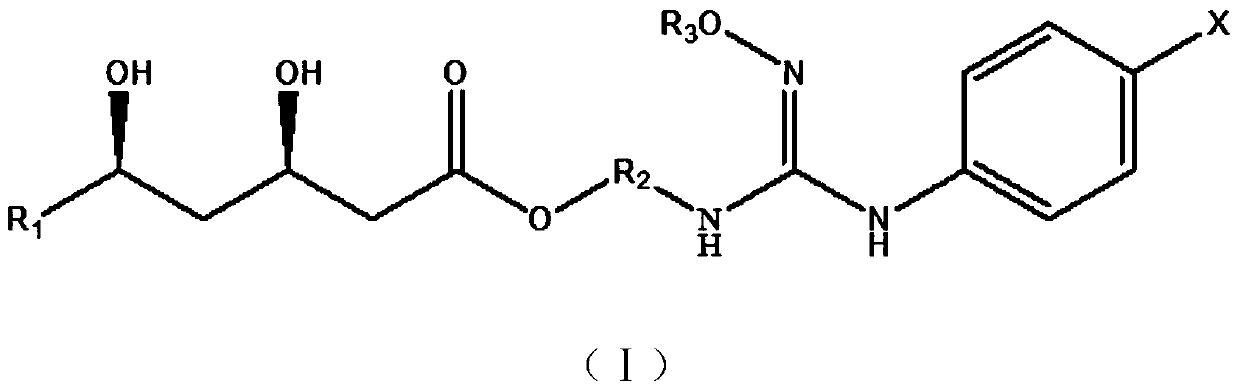
Image
Examples
Embodiment 1
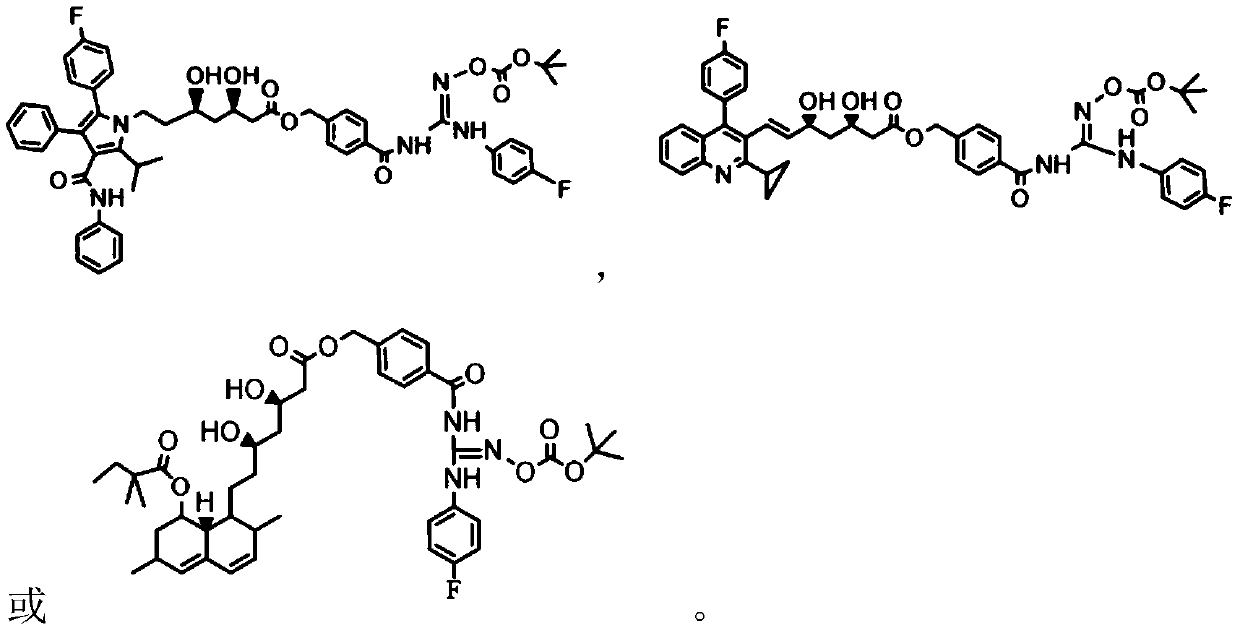
[0038] Example 1: (3R,5R)-7-[5-isopropyl-3-phenyl-2-(4-fluorophenyl)-4-(anicarboyl)pyrrole-1-]-3, 5-Dihydroxyheptanoic acid-{4-{N'-[(tert-butoxycarbonyl)oxy]-N-(4-chlorophenyl)formamidoyl}carbamoyl}benzyl ester
[0039]
[0040] Step 1: N-(4-Chlorophenyl)-N'-benzoylthiourea is compound 2a
[0041]
[0042] At room temperature, dissolve 18.42g of NH4SCN in 250ml of acetone, add 29.5g of PhCOCl at one time under stirring, heat up to reflux for 15min, stop heating, cool to room temperature, slowly add 20.58g of 4-chloroaniline in batches, and continue stirring until the reaction Completely, cool to room temperature. The reaction solution was poured into 700 g of crushed ice, filtered after the ice melted, the filtrate was discarded, the filter cake was recrystallized with acetone, and dried to obtain 41.5 g of white to off-white needle crystals with a yield of 88.5%. mp: 144.5-145.4
[0043] Step 2: 1-(4-Chlorophenyl)-thiourea is compound 3a
[0044]
[0045] Add 41.0 ...
Embodiment 2
[0065] Example 2: (3R,5S)-7-[2-cyclopropyl-4-(4-fluorophenyl)quinoline-3-]-3,5-dihydroxy-6(E)-heptenoic acid -{4-{N'-[(tert-butoxycarbonyl)oxyl]-N-(4-chlorophenyl)formamidoyl}carbamoyl}benzyl ester is the compound NO-6a-Pita
[0066]
[0067] Dissolve 0.4g of 6a and 0.8g of pitavastatin calcium in 10ml of DMF, add 0.8g of KI, and stir at room temperature for 48h. The reaction solution was poured into 50 ml of deionized water, extracted with ethyl acetate, and then the EA layer was washed with saturated brine, dried over anhydrous magnesium sulfate, filtered to remove the filter cake, and the filtrate was concentrated to obtain a crude product. Column chromatography separation to obtain the target product NO-6a-Pita
[0068] M+1: 823.1, 1H-NMR (DMSO-d6, 400MHz): δ8.02 (d, 2H, J = 8.4), 7.85 (d, 1H, J = 8.4), 7.64 (d, 1H), 7.60 ( d, 2H, J=8.4), 7.50(d, 2H, J=8.8), 7.41(d, 2H, J=8.8), 7.37-7.23(m, 6H), 6.51(d, 1H), 5.61(dd , 1H), 5.22(s, 2H), 4.88(d, 1H), 4.81(d, 1H), 4.13(...
Embodiment 3
[0069] Example 3: (3R, 5S)-7-[6-isopropyl-2-(N-methyl-N-methylsulfonylamino)-4-(4-chlorophenyl)pyrimidine-5- ]-3,5-Dihydroxy-6(E)-heptenoic acid-{4-{N'-[(tert-butoxycarbonyl)oxy]-N-(4-chlorophenyl)formamidoyl}amino Formyl}benzyl ester is the compound NO-6a-Rosu
[0070]
[0071] Dissolve 0.4g of 6a and 0.8g of rosuvastatin calcium in 10ml of DMF, add 0.8g of KI, and stir at room temperature for 48h. The reaction solution was poured into 50 ml of deionized water, extracted with ethyl acetate, and then the EA layer was washed with saturated brine, dried over anhydrous magnesium sulfate, filtered to remove the filter cake, and the filtrate was concentrated to obtain a crude product. Column chromatography separation to obtain the target product NO-6a-Ato
[0072] M+1: 883.3, 1H-NMR (DMSO-d6, 400MHz): δ8.03(d, 2H), 7.70(dd, 2H) 7.59(d, 2H), 7.50(d, 2H), 7.41(d, 2H), 7.26(dd, 2H), 6.52(d, 1H), 5.51(dd, 1H), 5.21(s, 2H), 4.97(d, 1H), 4.85(d, 1H), 4.20(m, 1H ), 3.90(m, 1H), 3.5...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com