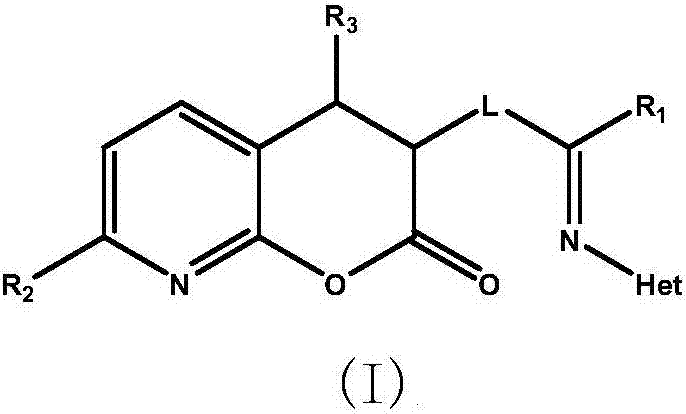
Medicine for treatment of cholecystitis and applications thereof
A technology of pharmacy, compounds, applied in the field of medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
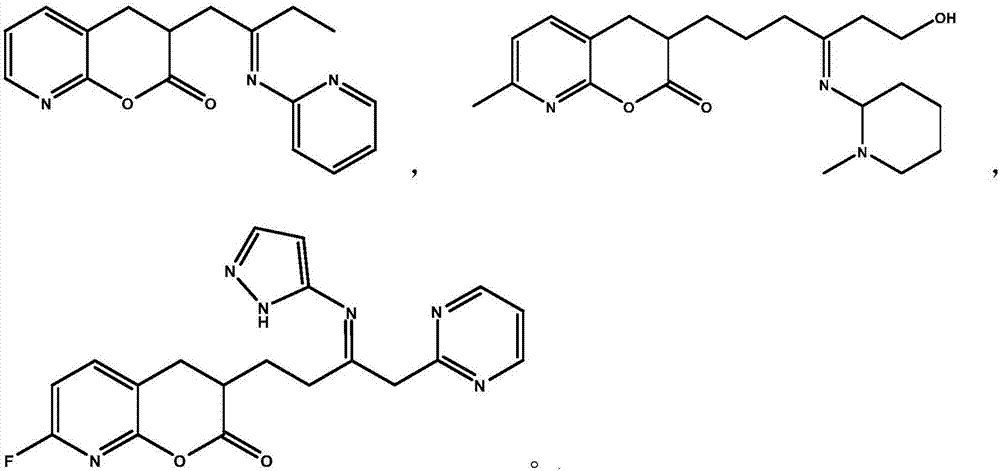
[0041] Example 1: 3-[2-(pyridin-2-ylimino)butyl]-3,4-dihydro-2H-pyran[2,3-b]pyridin-2-one (Compound A)
[0042]
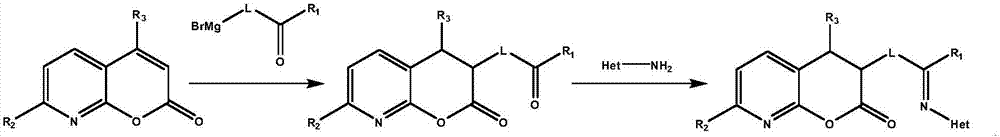
[0043] Dissolve 1-bromobutan-2-one (1.6g, 11mmol) in 50mL of anhydrous ether, and drop it into a flask containing freshly planed magnesium chips (0.29g, 12mmol) and 1 grain of iodine under stirring, for about 20 minutes. Complete, then stir and reflux until the magnesium chips are basically completely dissolved, stop heating, wait for the Grignard reagent to cool to 30-40°C, add 2H-pyran[2,3-b]pyridin-2-one (1.5g, 10mmol), Then react at 30-40°C for 3 hours, after the reaction, cool the reaction solution to room temperature, and then add saturated NaCO 3 Aqueous solution (50mL), extracted with petroleum ether (50mL×3), anhydrous CaCl 2 After drying, the solvent was distilled off under reduced pressure, the crude product was recrystallized with ethanol, and dried under vacuum at 50°C to obtain a light yellow solid 3-(2-oxobutyl)-3,4-dihydro-2H-pyran[2, 3-b] Pyri...
Embodiment 2
[0050] Example 2: 3-[6-Hydroxy-4-(1-methylpiperidin-2-ylimino)hexyl]-7-methyl-3,4-dihydro-2H-pyran[2,3 -b] pyridin-2-one (Compound B)
[0051]
[0052] According to the method of Example 1, replace 1-bromobutan-2-one with 6-bromo-1-hydroxyhexan-3-one, and use 7-methyl-2H-pyrano[2,3-b]pyridine -2-one in place of 2H-pyrano[2,3-b]pyridin-2-one and 1-methylpiperidin-2-amine in place of pyridin-2-amine gave a white solid 3-[6-hydroxy- 4-(1-Methylpiperidin-2-ylimino)hexyl]-7-methyl-3,4-dihydro-2H-pyran[2,3-b]pyridin-2-one, two steps Overall yield 59%.
[0053] ESI-MS: 374.24[M+H] +
[0054] Elemental analysis: theoretical value / measured value, C(67.53 / 67.46), H(8.37 / 8.44), N(11.25 / 11.36), O(12.85 / 12.74)
[0055] 1 H NMR (400MHz, CDCl 3 )δ7.55(d,1H),6.03(d,1H),4.77(s,1H),3.95(t,2H),2.90(d,2H),2.70(m,1H),2.52-2.56(m ,7H), 2.26-2.30(m,7H), 1.68(m,3H), 1.49-1.55(m,6H).
Embodiment 3
[0056] Example 3: 3-[3-(1H-pyrazol-5-ylimino)-4-(pyrimidin-2-yl)butyl)-7-fluoro-3,4-dihydro-2H-pyran [2,3-b]pyridin-2-one (Compound C)
[0057]
[0058] According to the method of Example 1, 6-bromo-1-hydroxyhexan-3-one was used instead of 1-bromobutan-2-one, and 7-fluoro-2H-pyrano[2,3-b]pyridine- Substitution of 2H-pyrano[2,3-b]pyridin-2-one by 2-keto and pyridin-2-amine by 1H-pyrazol-5-amine afforded 3-[3-(1H-pyrazole -5-ylimino)-4-(pyrimidin-2-yl)butyl)-7-fluoro-3,4-dihydro-2H-pyran[2,3-b]pyridin-2-one, two The overall yield was 51%.
[0059] ESI-MS: 381.14[M+H] +
[0060] Elemental analysis: theoretical value / measured value, C(59.99 / 59.81), H(4.50 / 4.59), F(4.99 / 4.93), N(22.09 / 22.01), O(8.41 / 8.66)
[0061] 1 H NMR (400MHz, CDCl 3 )δ12.51(s,1H),8.75(d,2H),7.95(d,1H),7.53-7.56(m,2H),6.36(d,1H),6.13(d,1H),2.96(d ,2H), 2.74(m,1H), 2.56(s,2H), 1.61(m,2H), 1.33(t,2H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com