Photovoltaic material as well as preparation method and application thereof
A photovoltaic material and difuran technology are applied in the field of naphthodifuran photovoltaic materials and their preparation, and achieve the effects of wide ultraviolet-visible absorption spectrum, high stability and stable performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] The preparation of 3,7-dinonyl-naphthodifuran-2,6-bis(2,5-diisooctyl-3,6-thienyl-2-pyrrolopyrrole dione (M1), including the following step:
[0038] Step 1) Under argon protection, 1,5-dihydroxynaphthalene (3.20g, 20.0mmol), 1-bromo-2-undecanone (11.2g, 45mmol), anhydrous potassium carbonate (8g, 58mmol) mixed solution In 100ml of anhydrous acetonitrile, heat and reflux for 4 hours; after the reaction is completed, cool to room temperature and pour into water, extract with dichloromethane, wash the mixed phase with water, dry over anhydrous magnesium sulfate, and then spin dry the organic phase to obtain the crude compound 1 , the crude product was rinsed through a silica gel column, and the eluent used in the rinse was a mixed solution of dichloromethane and petroleum ether with a volume ratio of 10:1 to obtain 7.4g of light yellow solid compound 1, and the reaction yield was 75%;
[0039] The characterization of the compound 1 is as follows:
[0040] GC-MS: m / z=497;...
Embodiment 2
[0059] The preparation of 3,7-dinonyl-naphthodifuran-2,6-bis(2,5-diisooctyl-3,6-thienyl-2-pyrrolopyrrole dione (M1), including the following step:
[0060] Step 1) Under argon protection, 1,5-dihydroxynaphthalene (3.20g, 20.0mmol), 1-bromo-2-undecanone (12.44g, 50mmol), and anhydrous potassium carbonate (4.14g, 30mmol) were mixed Dissolve in 100ml of anhydrous acetonitrile, heat and reflux for 3.5 hours; after the reaction is completed, cool to room temperature and pour into water, extract with dichloromethane, wash the mixed phase with water, dry over anhydrous magnesium sulfate, and spin dry the organic phase to obtain compound 1 The crude product was washed through a silica gel column, and the eluent used in the washing was a mixture of dichloromethane and petroleum ether with a volume ratio of 8:1 to obtain 7.5 g of light yellow solid compound 1 with a reaction yield of 77%;
[0061] The characterization of the compound 1 is as follows:
[0062] GC-MS: m / z=497;
[0063]...
Embodiment 3
[0081] The preparation of 3,7-dinonyl-naphthodifuran-2,6-bis(2,5-diisooctyl-3,6-thienyl-2-pyrrolopyrrole dione (M1), including the following step:
[0082] Step 1) Under argon protection, 1,5-dihydroxynaphthalene (3.20g, 20.0mmol), 1-bromo-2-undecanone (11.94g, 48mmol), and anhydrous potassium carbonate (6.07g, 44mmol) were mixed Dissolve in 100ml of anhydrous acetonitrile, heat and reflux for 4.5 hours; after the reaction is completed, cool to room temperature and pour into water, extract with dichloromethane, wash the mixed phase with water, dry over anhydrous magnesium sulfate, and spin dry the organic phase to obtain compound 1 The crude product was washed through a silica gel column, and the eluent used in the washing was a mixture of dichloromethane and n-hexane with a volume ratio of 9:1 to obtain 7.3 g of light yellow solid compound 1 with a reaction yield of 74%;
[0083] The characterization of the compound 1 is as follows:
[0084] GC-MS: m / z=497;
[0085] 1 H N...
PUM
| Property | Measurement | Unit |
|---|---|---|
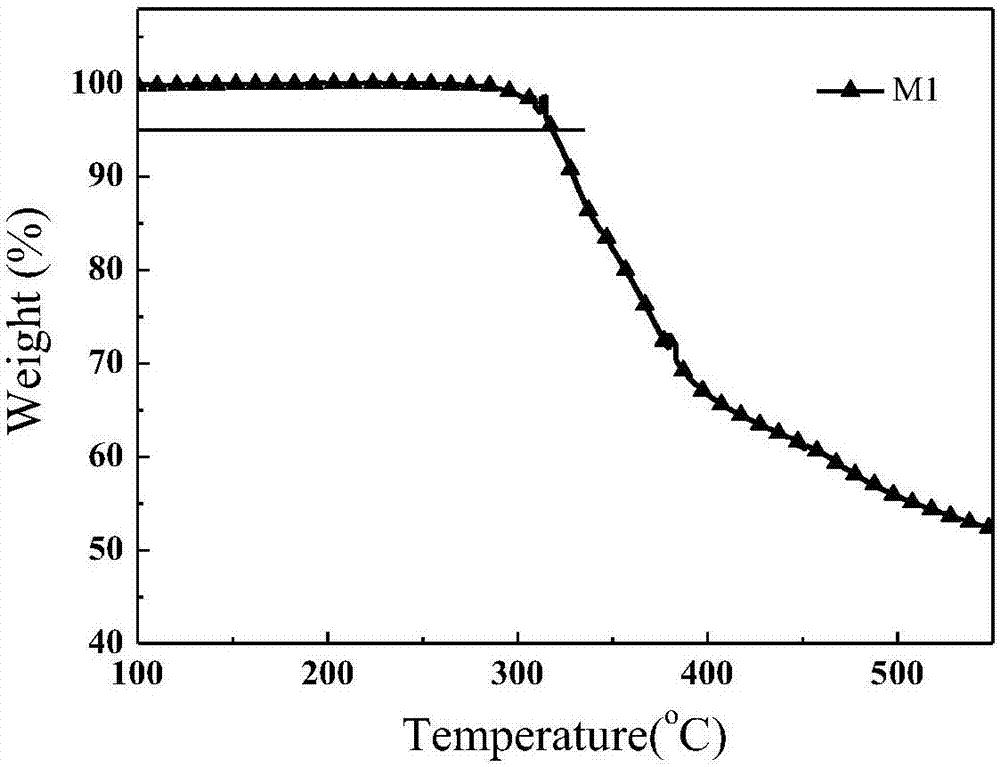
| Thermal decomposition temperature | aaaaa | aaaaa |
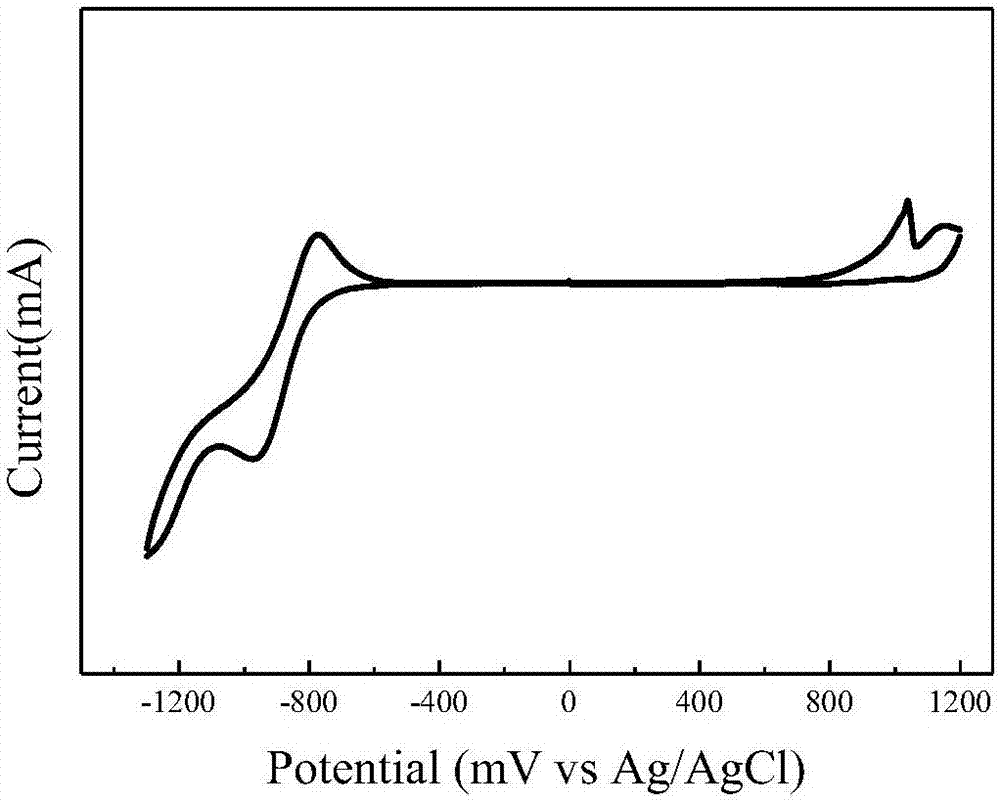
| Oxidation potential | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com