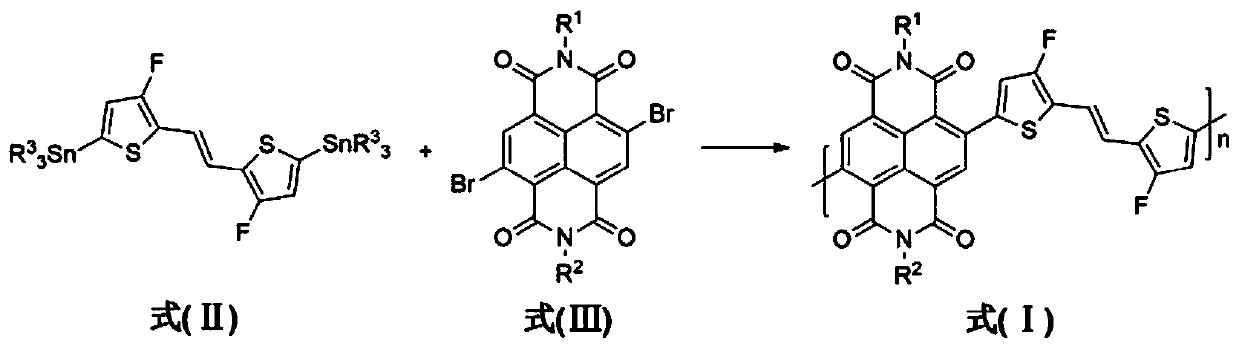
A kind of naphthalimide-fluorinated dithienylethylene conjugated polymer and its preparation method and application
A technology of fluorinated dithiophene ethylene and conjugated polymers, which is applied in semiconductor/solid-state device manufacturing, electric solid-state devices, semiconductor devices, etc., can solve problems such as poor device stability, achieve low frontier orbital energy levels, and reduce reaction yields. The effect of high rate and high polymer molecular weight
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
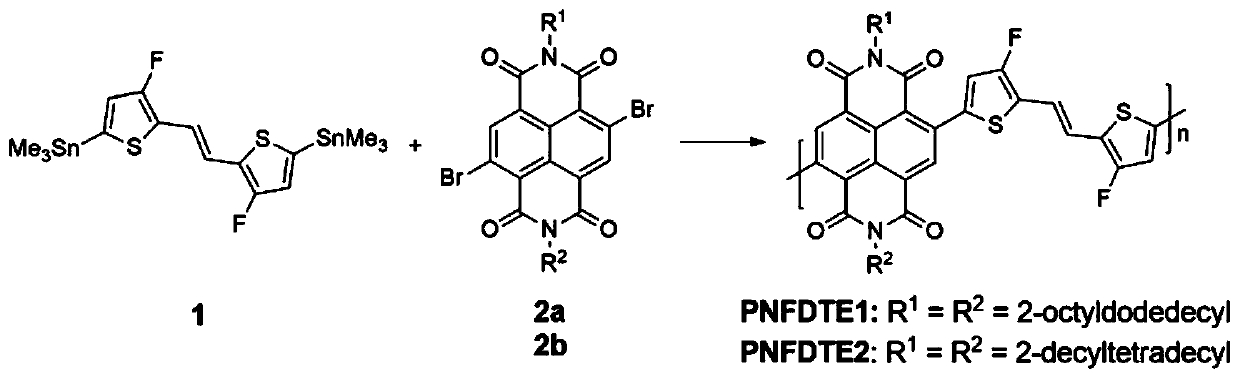
[0048] Poly{N,N-bis(2-octyldodecyl)-1,4,5,8-naphthalimide-2,6-(trans-1,2-bis(3-fluorothiophene-2 -base) vinyl)-2,5-diyl} is abbreviated as polymer PNFDTE1 below, that is, R in formula (I) 1 =R 2 = 2-octyldodecyl, the synthetic route is as figure 2 As shown, the preparation method is as follows:
[0049] Mix (trans)-1,2-bis(3-fluoro-5-trimethyltin-thiophen-2-yl)ethylene (110.8mg, 0.20mmol) and 2,6-dibromo-N,N-di (2-Octyldodecyl)-1,4,5,8-naphthalimide (197.0mg, 0.2mmol), tris(dibenzylideneacetone)dipalladium (9mg), tris(o-tolyl ) Phosphine (24.6mg) and chlorobenzene (5.0mL) were added to the reaction flask, three freeze-pump-thaw cycles were performed in argon to remove oxygen, and the reaction mixture was heated to 110°C under argon protection for 72h. After cooling, 200 mL of methanol / 6M hydrochloric acid mixture (v / v 20:1) was added, stirred at room temperature for 2 h, and filtered. The resulting solid was extracted with a Soxhlet extractor. The extraction solvents we...
Embodiment 2
[0055] Poly{N,N-bis(2-decyltetradecyl)-1,4,5,8-naphthalimide-2,6-(trans-1,2-bis(3-fluorothiophene-2 -base) ethylene) -2,5-diyl} hereinafter abbreviated as polymer PNFDTE2, that is, R in formula (I) 1 =R 2 =2-decyltetradecyl, the synthetic route is as figure 2 As shown, the preparation method is as follows:
[0056] Mix (trans)-1,2-bis(3-fluoro-5-trimethyltin-thiophen-2-yl)ethylene (110.8mg, 0.20mmol) and 2,6-dibromo-N,N-di (2-decyltetradecyl)-1,4,5,8-naphthalimide (219.5mg, 0.20mmol), tris(dibenzylideneacetone)dipalladium (9mg), tris(o-tolyl ) Phosphine (24.6mg) and chlorobenzene (5.0mL) were added to the reaction flask, three freeze-pump-thaw cycles were performed in argon to remove oxygen, and the reaction mixture was heated to 110°C under argon protection for 72h. After cooling, 200 mL of methanol / 6M hydrochloric acid mixture (v / v 20:1) was added, stirred at room temperature for 2 h, and filtered. The resulting solid was extracted with a Soxhlet extractor. The extrac...
Embodiment 3
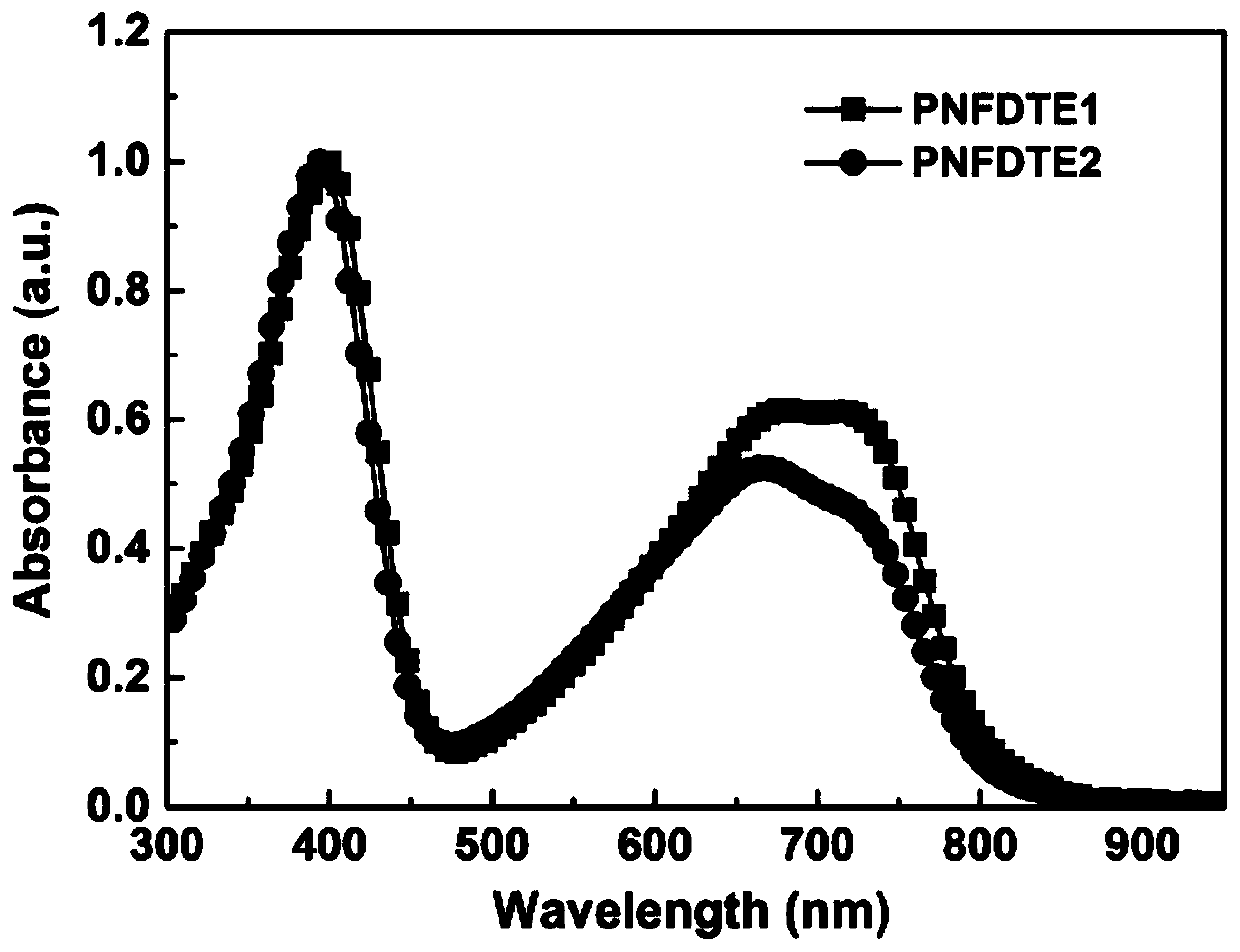
[0062] Experiments on Spectral Properties of Polymer PNFDTE1 and Polymer PNFDTE2
[0063] image 3 and Figure 4 Be respectively the ultraviolet-visible absorption spectrogram of polymer PNFDTE1 and polymer PNFDTE2 chlorobenzene solution and film prepared by embodiment 1 and embodiment 2, from image 3 It can be seen that there are two absorption bands in this type of polymer, the secondary absorption band is a high energy band, and its absorption is at 300 to 470 nm, and the main absorption band is a low energy band, and its absorption is at 470 to 810 nm. Stronger low-energy band absorption indicates stronger intramolecular charge transfer within the polymer molecule. Depend on Figure 4 It can be seen that the absorption curve of the film has a certain degree of red shift compared with that of the solution, and the main absorption band is a low energy band, and the absorption is from 470 to 850 or 870 nanometers.
PUM
| Property | Measurement | Unit |
|---|---|---|
| thermal decomposition temperature | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com