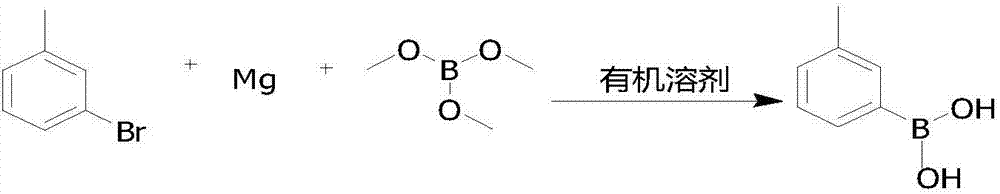
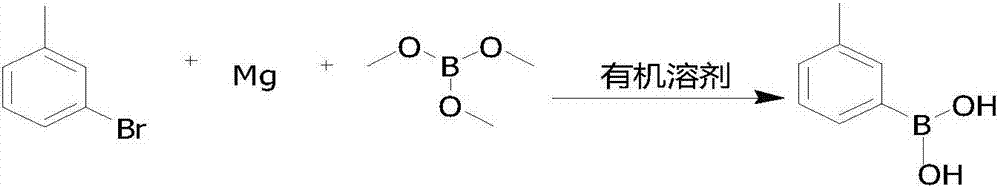
Preparation method for 3-carboxyphenylboronic acid
A technology of carboxyphenylboronic acid and methylbenzeneboronic acid, which is applied in the field of preparation of 3-carboxyphenylboronic acid, can solve the problems of unsuitability for industrial production and high cost of raw materials, and achieve the effects of low cost, simple operation, and avoiding high-temperature reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Add 0.05mol (0.1eq) m-bromotoluene, 0.55mol (1.1eq) magnesium chips, several grains of elemental iodine, 300ml THF into the reaction flask, stir, heat up to reflux, Grignard triggers, continue to drop m-bromotoluene under reflux 0.45 mol (0.9 eq). After the dropwise addition, reflux and heat preservation reaction, when the reaction time is up, lower the temperature to -60.0°C, start to add 1.0mol (2.0eq) of trimethyl borate dropwise, after the dropwise addition is complete, keep the heat preservation reaction until the raw materials are reacted, heat up, add water, and dropwise add hydrochloric acid Hydrolyze, adjust the pH to 1-2, heat preservation reaction, the reaction time is up, rotary evaporation, draining, beating and purification, drying to obtain 0.44mol of white solid 3-methylphenylboronic acid, moisture 0.05%, purity 98.64%, yield is 88.0%.
[0025] Drop into 120ml of aqueous sodium hydroxide solution (30%) in the reaction flask, 0.44mol of the above-mention...
Embodiment 2
[0027] Add 0.10mol (0.1eq) m-bromotoluene, 1.1mol (1.1eq) magnesium chips, a few grains of elemental iodine, 800mlTHF into the reaction flask, stir, heat up to reflux, Grignard triggers, and continue to drop m-bromotoluene 0.90ml under the reflux state. mol (0.9eq), after the dropwise addition, reflux and keep warm for reaction, when the reaction time is up, cool down to -50.0°C, start to add 2.2mol (2.2eq) of trimethyl borate dropwise, after the dropwise addition is complete, keep warm for reaction until the raw materials are reacted, then raise the temperature , add water, add hydrochloric acid dropwise for hydrolysis, adjust pH to 1-2, keep warm for reaction, when the reaction time is up, rotary evaporate, drain, beat and purify, and dry to obtain 0.90mol of white solid 3-methylphenylboronic acid, moisture 0.03%, purity 98.86%, yield 90.0%.
[0028] Drop into 250ml of aqueous sodium hydroxide solution (30%) in the reaction bottle, 0.90mol of the above-mentioned 3-methylphen...
Embodiment 3
[0030] Add 0.10mol (0.1eq) m-bromotoluene, 1.2mol (1.2eq) magnesium chips, several grains of elemental iodine, 800mlTHF into the reaction flask, stir, heat up to reflux, Grignard triggers, and continue to drop m-bromotoluene 0.90ml in the reflux state. mol (0.9eq), after the dropwise addition, reflux and keep warm for reaction, when the reaction time is up, cool down to -60.0°C, start to add 2.0mol (2.0eq) of trimethyl borate dropwise, after the dropwise addition is complete, keep warm for reaction until the raw materials are reacted, then raise the temperature , add water, add hydrochloric acid dropwise for hydrolysis, adjust pH to 1-2, keep warm for reaction, when the reaction time is up, rotary evaporate, drain, beat and purify, and dry to obtain 0.87mol of white solid 3-methylphenylboronic acid, moisture 0.03%, purity 98.57%, and the yield was 87.0%.
[0031] Drop into 300ml of aqueous sodium hydroxide solution (30%) in the reaction flask, 0.87mol of the above-mentioned 3-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com