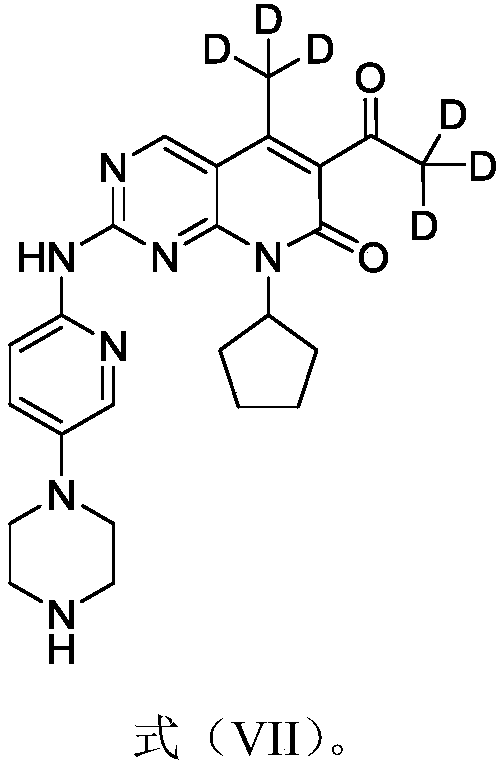
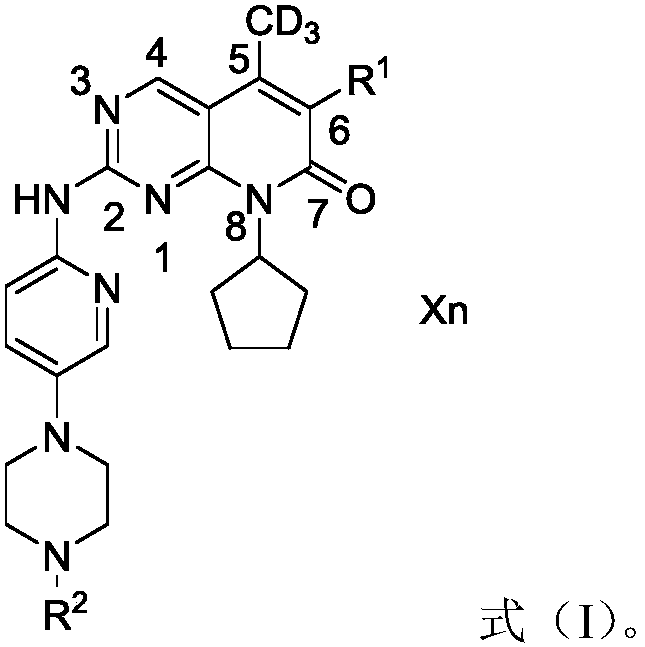
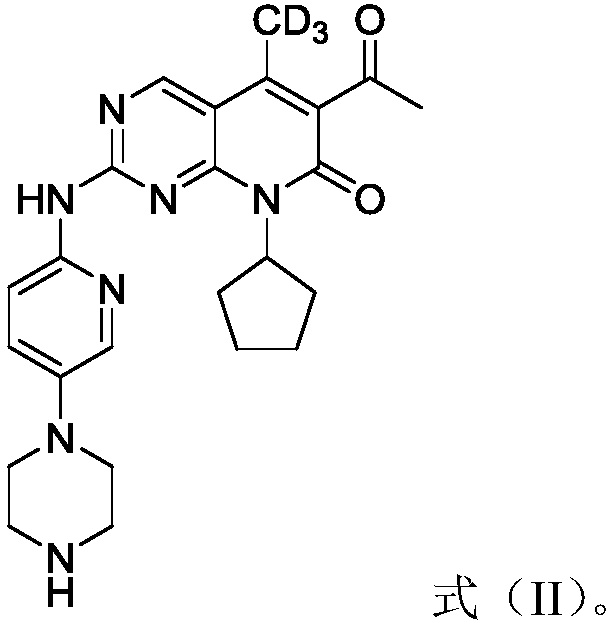
Derivatives, preparation method and application of deuterated palbociclib
A derivative and deuterium technology, which is applied in the field of pharmaceutical compounds, can solve the problems of high cost, poor compound stability, and low deuterium rate, and achieve the effects of solving instability, improving curative effect, increasing solubility and dissolution rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] The present embodiment prepares the Palbociclib derivative shown in formula (V), and its synthetic route is as follows:
[0050]
[0051] The present embodiment prepares the Palbociclib derivative shown in formula (V), comprises the following steps:
[0052] 1) Synthesis of the compound shown in formula (IV): Take 36g of compound A and add it to 250ml of tetrahydrofuran, and cool the system to 0°C; under nitrogen protection, slowly add 240mL of tetrahydrofuran solution of isopropylmagnesium chloride (1M), dropwise After the addition was complete, the system was warmed up to room temperature and continued to react for 1 h; then 35 g of compound B was added, and the reaction was continued overnight, and saturated NH 4 Cl solution quenched the reaction, extracted three times with ethyl acetate, combined the organic phases, dried and concentrated to obtain the compound shown in 44g formula (IV);
[0053] The nuclear magnetic information of compound shown in formula (IV)...
Embodiment 2
[0058] This embodiment prepares the deuterated Palbociclib derivative shown in formula (VI), and its synthetic route is as follows:
[0059] The preparation of deuterated Palbociclib derivatives shown in formula (VI) in this embodiment comprises the following steps:
[0060] 1) prepare the Palbociclib derivative shown in formula (IV) according to the method of Example 1;
[0061] 2) prepare the Palbociclib derivative shown in formula (V) according to the method of Example 1;
[0062] 3) Under nitrogen protection, take 450mg of the compound shown in formula (V), 240mg of vinyl n-butyl ether, 10mg of Pd(OAc) 2 , 50 mg of dppf, and 160 mg of DIEPA were added to 20 mL of n-BuOH, and the system was heated to 95° C. for 20 h; after the reaction, the system was concentrated and purified by column chromatography to obtain 290 mg of the compound represented by formula (VI).
[0063] The nuclear magnetic resonance information of compound shown in formula (VI) is: 1 H NMR (400MHz, C...
Embodiment 3
[0065] The present embodiment prepares the deuterated Palbociclib derivative shown in formula (II), and its structural formula is as follows:
[0066]
[0067] The preparation method of the deuterated Palbociclib derivative shown in formula (II) comprises the following steps: take 300 mg of the compound shown in formula (VI) prepared in Example 2, add it to 20 mL of dichloromethane, add about 1 mL of concentrated hydrochloric acid dropwise at room temperature, Stir overnight at room temperature, the system precipitates a viscous yellow solid, add saturated NaHCO 3solution, adjusted until the system was weakly alkaline, the system was extracted with dichloromethane, the organic phase was dried, and concentrated to obtain 210 mg of the deuterated Palbociclib derivative shown in formula (II);
[0068] The nuclear magnetic resonance information of the deuterated Palbociclib derivative shown in formula (II) is: 1 H NMR (400MHz, CDCl 3 ): δ8.82(s,1H),8.21(s,1H),8.17-8.15(d,1H,J...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com