Method for preparing dichloro monoalkyl phosphine
A technology of dichloro-alkylphosphine and dichloro-alkylaluminum, which is applied in the field of metal-organic compound preparation, can solve the problems of low product purity, non-commercialization, and low yield, so as to reduce solid waste residue and increase yield , the effect of increasing the yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1 2
[0047] Embodiment 1 Dichloromonomethylphosphine aluminum trichloride complex MePCl 2 ·AlCl 3 Preparation of:
[0048] A five-necked glass flask is used as a reaction bottle equipped with an electric stirrer, a thermometer, two dropping funnels, and a vent port connected to a Schlenk Line. After the flask is replaced with pure nitrogen, it is filled with nitrogen to normal pressure.
[0049] Dichloromonomethylaluminum 100g (0.886mol) was dissolved in 660g n-octane at room temperature, and placed in a dropping funnel. Phosphorus trichloride 121.6g was diluted with 243g n-octane, and placed in another dropping funnel. 50ml of n-octane was preset at the bottom of the reaction bottle.
[0050] Stir at room temperature and start to add dichloromonomethylaluminum and phosphorus trichloride n-octane solution synchronously. At this time, the temperature in the reaction bottle rises automatically. Complete dichloromonomethylaluminum n-octane solution and phosphorus trichloride n-oct...
Embodiment 2
[0054] Phosphorus trichloride binary phosphorus aluminum exchange product (3MePCl of embodiment 2 methyl sesquichloride aluminum 2 )·(2·AlCl 3 ) preparation:
[0055] The reaction apparatus is the same as in Example 1, 100 g (0.487 mol) of methyl aluminum sesquichloride is diluted with 1000 g of n-octane, and placed in a dropping funnel. Phosphorus trichloride 301g (2.25mol, excessive phosphorus trichloride) is diluted with 600g n-octane, and placed in another dropping funnel. 50ml of n-octane was preset at the bottom of the reaction bottle.
[0056] Stir at room temperature and start to add methyl sesquichloride and phosphorus trichloride n-octane solution synchronously. At this time, the temperature in the reaction bottle rises automatically. After the addition of methyl aluminum sesquichloride n-octane solution and phosphorus trichloride n-octane solution, stir at room temperature for 24 hours or raise the temperature to 60° C. and stir for 5 hours to stop. Change to va...
Embodiment 3
[0057] Example 3 From MePCl 2 ·AlCl 3 Preparation of dichloromonomethylphosphine:
[0058] MePCl 2 ·AlCl 3 +NaCl→MePCl 2 +NaAlCl 4
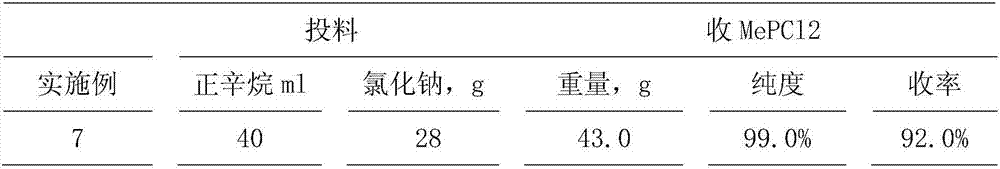
[0059] Get embodiment 1 gained and contain 70.8% MePCl 2 ·AlCl 3 Add 28.1 g of 200-mesh sodium chloride to 141.2 g of n-octane mixture, stir and heat up to 125° C., stir at this temperature for 6 hours, cool and filter, and wash the filter residue in 3 batches with 100 ml of n-octane. Obtain 81g of filter residue, which is sodium tetrachloroaluminate containing sodium chloride; combine the filtrates, collect fractions at 79-81°C by fractional distillation, and obtain dichloromonomethylphosphine MePCl 2 42.8g, purity 99.4%, density 1.3005 (20°C), yield 91%. The n-octane recovery sleeve is used for the next batch of operations.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com