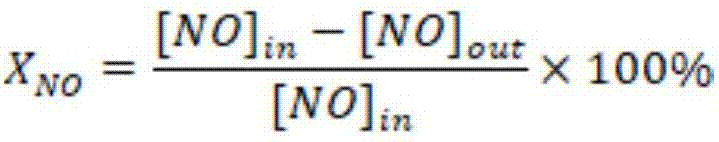Loaded tourmaline rare earth compound selective denitration catalyst
A denitrification catalyst and rare earth composite technology, which is applied in the field of selective denitration catalyst and its preparation, can solve the problems of not being able to meet the low temperature wide denitrification activity window, high selectivity and high water resistance and sulfur resistance, and achieve environmental protection, The effect of reducing energy consumption and cost, and reducing production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
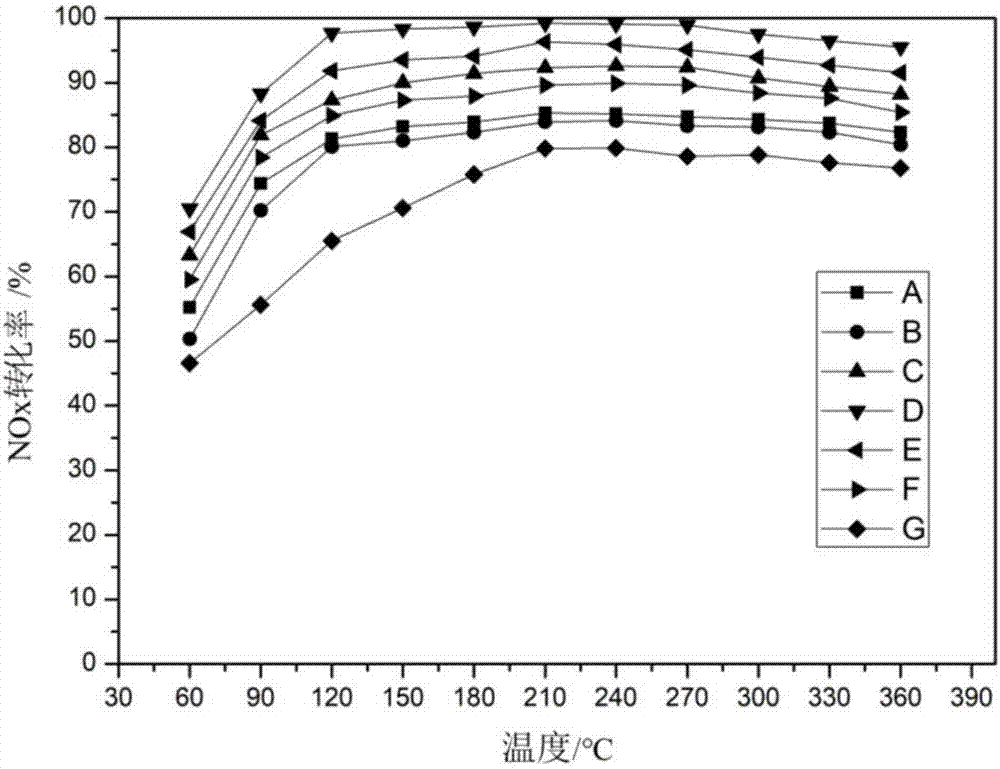
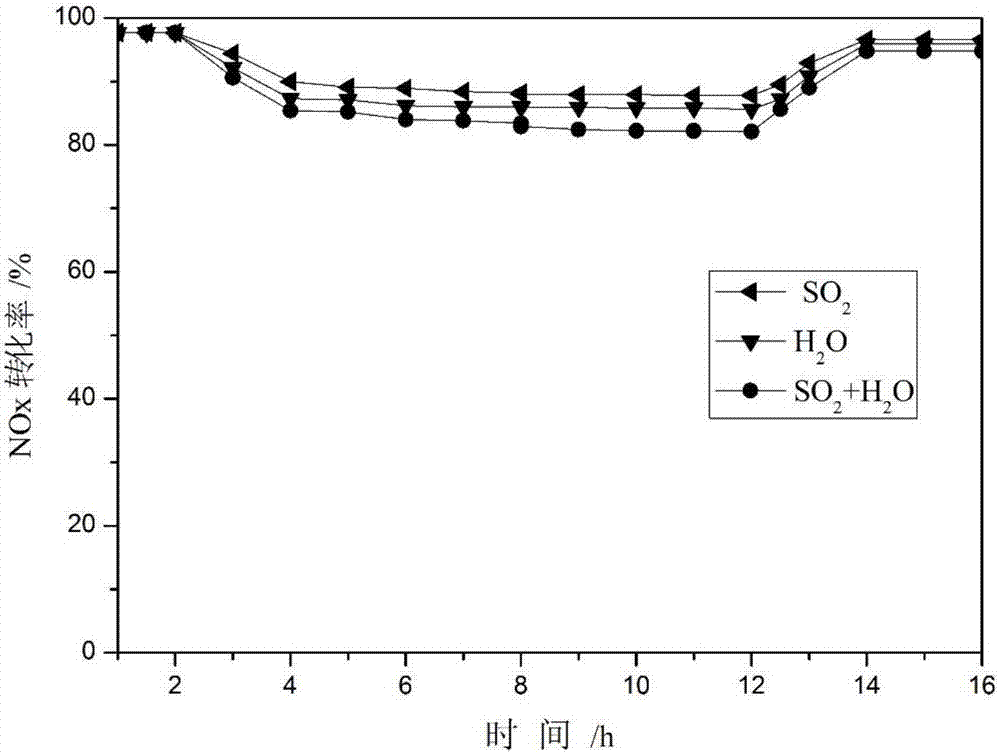
Image
Examples
Embodiment 1
[0040] 23.24 mL of manganese nitrate solution with a mass fraction of 50%, cerium nitrate hexahydrate (Ce(NO 3 ) 3 ·6H 2 O) 4.3414g and samarium nitrate hexahydrate (Sm(NO 3 ) 3 ·6H 2 O) 4.4467g was dissolved in 176.76mL deionized water to obtain a mixed solution, so that the molar ratio of Mn:Ce:Sm was 10:1:1, and the solution was mixed uniformly by continuous mechanical stirring at room temperature for 1h; then 0.4991g of the average particle Diameter is 65nm, iron content is 15.5wt.% iron tourmaline to add wherein, make it account for 1.0% of material gross mass, (the described material is manganese salt, cerium salt, samarium salt, tourmaline and carrier SBA-15. ) mechanically stirred for 1h; 22.3375g of SBA-15 carrier was added to the mixed solution, so that the carrier accounted for 45.0% of the total mass of the material, and mechanically stirred for 2h; 4mol / L ammonia solution was slowly added dropwise to the mixed solution at a rate of 2mL / min The solution was st...
Embodiment 2
[0042] 23.24mL of 50% manganese nitrate solution, 4.3414g of cerium nitrate and 4.4467g of samarium nitrate were dissolved in 176.76mL of deionized water to obtain a mixed solution, so that the molar ratio of Mn:Ce:Sm was 10:1:1, and continuously Stir mechanically for 1 hour to make the solution evenly mixed; then add 0.0624g ferrite tourmaline to make it account for 0.1% of the total mass of the material, and stir mechanically for 1 hour; add 22.2707g SBA-15 carrier to the mixed solution to make the carrier account for the total mass of the material 45.9% of the mass, mechanically stirred for 2h; 4mol / L ammonia solution was slowly added dropwise to the mixed solution at a rate of 2mL / min until the pH of the solution = 12, and mechanically stirred for 2h; stood for 24h to separate layers, and removed the supernatant , Transfer the precipitate to a hydrothermal kettle for 24 hours at 180°C. Suction filter and wash the hydrothermal product, put the filter cake in a drying box an...
Embodiment 3
[0044] Steps are as in Example 1, other conditions remain unchanged, change 50% manganese nitrate solution to 7.44mL, deionized water to 192.56mL, make the molar ratio of Mn:Ce:Sm be 3.2:1:1; tourmaline quality is 0.2999g , accounting for 1.0% of the total mass of the material; the mass of SBA-15 is 12.4770g, accounting for 45.0% of the total mass of the material. Catalyst C is prepared.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com