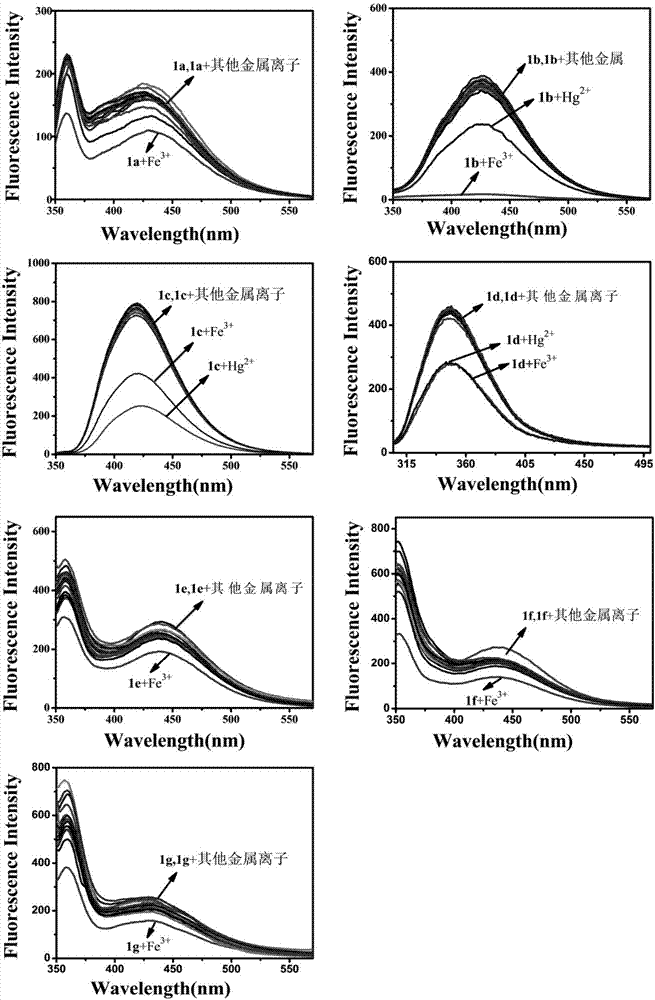
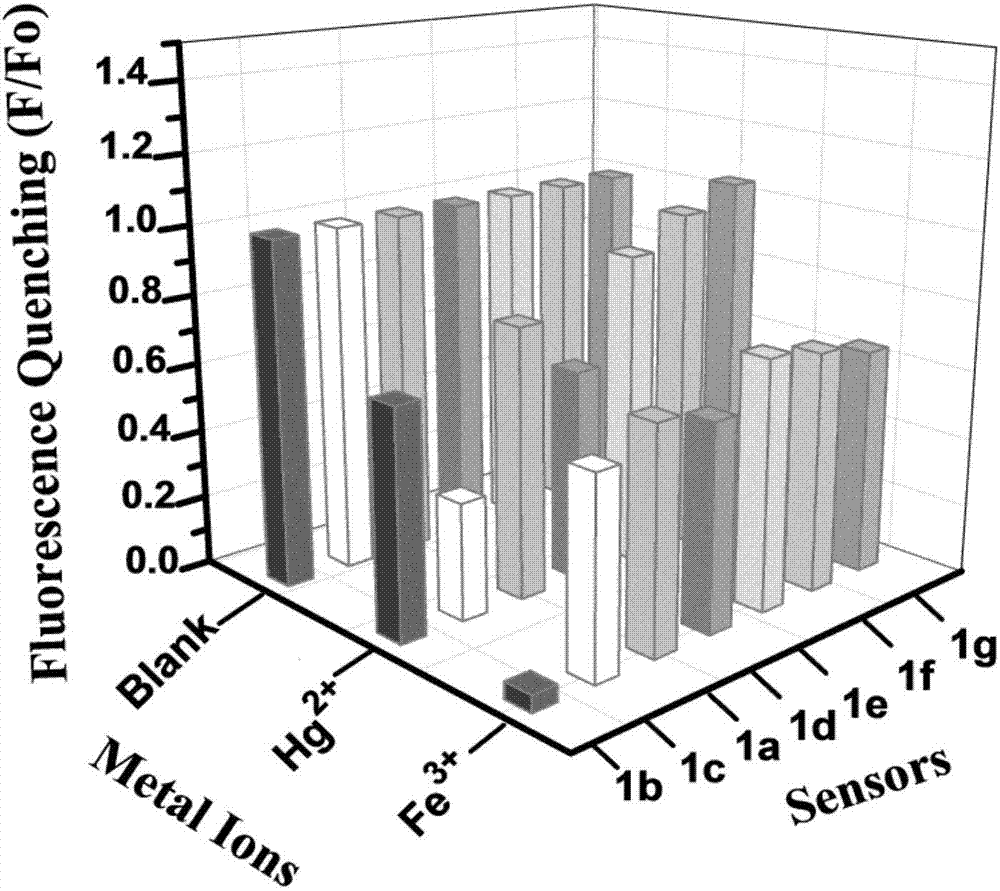
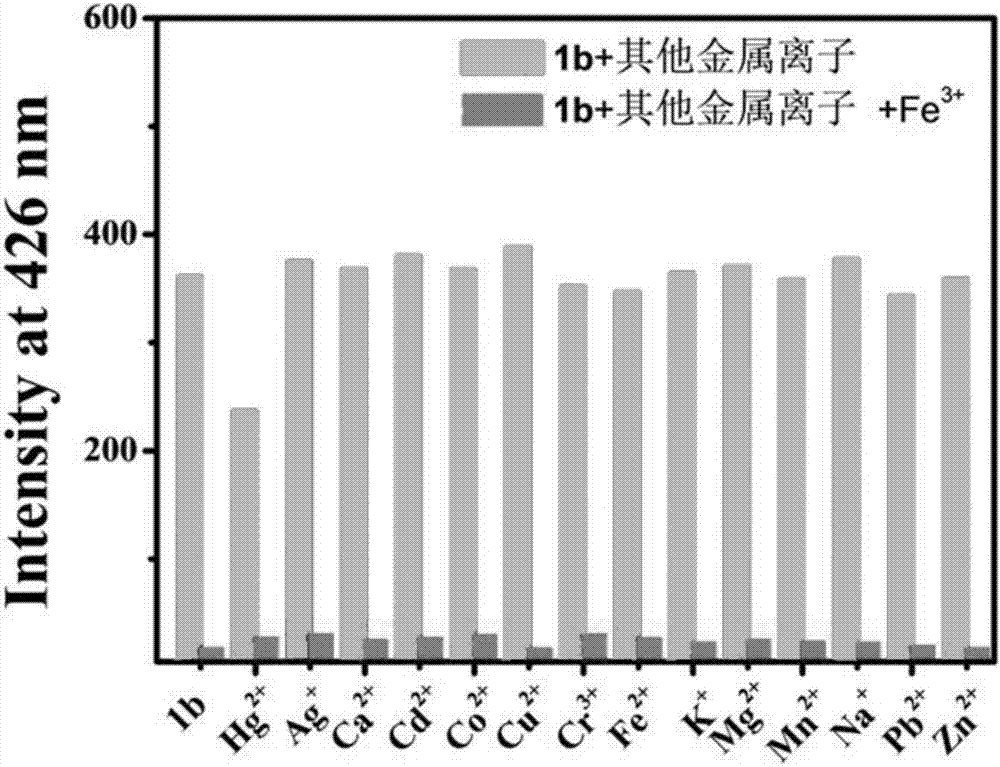
Benzoyl amide derivative fluorescence probes for identifying and detecting ferric ion and divalent mercury ion, preparation method and application thereof
A fluorescent probe and benzamide technology, applied in the field of fluorescent probes, can solve the problems of poor water solubility and low sensitivity, and achieve the effect of simple method, low cost and good application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Example 1 Preparation of 3,6-dithia-1,8-octanediamine
[0045] Dissolve 43mmol sodium ethoxide in 40mL absolute ethanol, cool to 10°C, add 24mmol cysteamine hydrochloride to the above solution under nitrogen protection, react for 1 hour, add 10mmol 1,2-dibromoethane, and react overnight at room temperature. After the reaction was completed, filter, spin evaporate, add diluted NaOH solution, and refrigerate overnight. Next, CH 2 Cl 2 Extracted, washed three times with water, dried over anhydrous sodium sulfate, rotary evaporated, and vacuum dried to obtain 3,6-dithia-1,8-octanediamine, weighing 1.2623g, yield 70.02%, the product structural formula is 1 H NMR, 13 Confirmed by CNMR and HRMS.
[0046] 1 H NMR (600MHz, CDCl 3 ):2.89(t, J=6.3Hz, 2H), 2.74(s, 2H), 2.66(t, J=6.3Hz, 2H); 13 C NMR (147MHz, CD 3 OD): 41.53, 35.45, 32.71; HRMS (ESI) m / z calcd for C 6 h 16 N 2 S 2 ,[M+H] + 181.08, found 181.0810.
Embodiment 2
[0047] Synthesis and identification data of embodiment 2 benzamide derivative fluorescent probe 1a
[0048] Dissolve 5.16mmol of benzoic acid in 50mL of dichloromethane, add EDCI (5mmol) and HOBt (5mmol) under stirring and cooling with ice water, stir and activate for 0.5-1h. Then 2 mmol of 3,6-dithia-1,8-octanediamine (obtained from Example 1) was added, and stirred overnight at room temperature. rotary steaming, CH 2 Cl 2 Extraction, successively with saturated NaHCO 3 , saturated NaCl, the organic phase was washed with water, and dried over anhydrous sodium sulfate. Rotary evaporation, the thick product is purified by silica column chromatography, and vacuum-dried to obtain fluorescent probe 1a, weighing 0.4913g, yield 63.3%, and the product structural formula is 1 H NMR, 13 Confirmed by C NMR and HRMS.
[0049] 1 H NMR (600MHz, DMSO-d 6 ):12.48(s, J=6.24Hz, 2H), 2.73(s, 4H), 2.66(t, J=6.24Hz, 4H); 13 C NMR (150MHz, CDCl 3 ):167.54,134.08,131.38,128.37,126.79,39.1...
Embodiment 3
[0052] Example 3 Synthesis and identification data of benzamide derivative fluorescent probe 1b
[0053] Dissolve 2.8mmol salicylic acid in 50mL dichloromethane, add EDCI (4mmol) and HOBt (4mmol) under stirring and cooling with ice water, stir and activate for 0.5-1h. Then 1 mmol of 3,6-dithia-1,8-octanediamine (obtained from Example 1) was added, and stirred overnight at room temperature. rotary steaming, CH 2 Cl 2 Extraction, successively with saturated NaHCO 3 , saturated NaCl, the organic phase was washed with water, and dried over anhydrous sodium sulfate. Rotary evaporation, the crude product is purified by silica column chromatography, and vacuum-dried to obtain fluorescent probe 1b with a yield of 46.9%, and the product structural formula is as follows: 1 H NMR, 13 Confirmed by C NMR and HRMS.
[0054] 1 H NMR (600MHz, DMSO-d 6 ):12.48(s,2H),8.95(t,J=5.6Hz,2H),7.82(d,J=7.9Hz,2H),7.39(t,J=8.1Hz,2H),6.93–6.85(m ,4H),3.48(q,J=6.6Hz,4H),2.78(s,4H),2.74(t,J=7.1Hz,4...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap