Method for preparing 4-cypro methylene phenylboronic acid
A technology of cyclopropyl methylene benzene boronic acid and cyclopropyl methylene chlorobenzene, applied in the field of preparing 4-cyclopropyl methylene benzene boronic acid, can solve the problem of low yield, high price of bromobenzene, and unsuitability for large-scale industrialization Problems such as producing 4-cyclopropyl methylene benzene boronic acid, to achieve the effect of reducing preparation cost and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
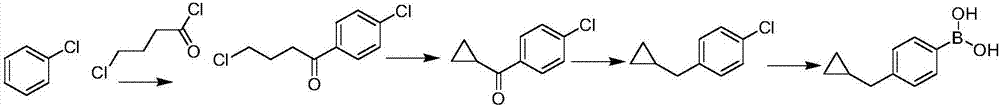
[0025] (1) Friedel-Crafts acylation reaction: 10L glass reactor equipped with mechanical stirring and thermometer, under the protection of nitrogen, add 1000g of chlorobenzene and 1000g of 4-chlorobutyryl chloride, cool to -5°C, add trichloride in batches under stirring Aluminum 1042g, after the reaction is complete, add the system to 10L of ice water, extract and separate layers with n-hexane, concentrate the organic layer to dryness, and recover chlorobenzene with an oil pump, leaving 4-chlorobutyrylchlorobenzene as the residue.
[0026] (2) Ring closure reaction: 20L glass reactor equipped with mechanical stirring and thermometer, add 6720g of ethanol under nitrogen protection, add 1440g of sodium hydroxide in batches under stirring, cool down to below 20°C after dissolving and add 4-chlorobutyryl dropwise Chlorobenzene, after dropping, keep warm overnight. The system was distilled under reduced pressure at 60°C until there was no solvent, water and n-hexane were added to e...
Embodiment 2
[0030] (1) Friedel-Crafts acylation reaction: a 10L glass reactor equipped with a mechanical stirrer and a thermometer, add 5000g of chlorobenzene and 1000g of 4-chlorobutyryl chloride under nitrogen protection, cool down to -5°C, and add the acylating reagent in batches under stirring 1042g of aluminum trichloride, after the reaction is completed, add the system to 10L of ice water, extract and separate layers with n-hexane, concentrate the organic layer to dryness, and recover chlorobenzene with an oil pump, and the residue is 4-chlorobutyryl chlorobenzene.
[0031] (2) Ring closure reaction: 20L glass reactor equipped with mechanical stirring and thermometer, add 6720g of ethanol under nitrogen protection, add 1440g of sodium hydroxide in batches under stirring, cool down to below 20°C after dissolving and add 4-chlorobutyryl dropwise Chlorobenzene, after dropping, keep warm overnight. The system was distilled under reduced pressure at 60°C until there was no solvent, water...
Embodiment 3
[0035] (1) Friedel-Crafts acylation reaction: 10L glass reactor equipped with mechanical stirring and thermometer, under the protection of nitrogen, add 5000g of chlorobenzene and 1000g of 4-chlorobutyryl chloride, cool to -5°C, add acylating reagent in batches under stirring 1042g of aluminum trichloride, after the reaction is completed, add the system to 10L of ice water, extract and separate layers with n-hexane, concentrate the organic layer to dryness, and recover chlorobenzene with an oil pump, and the residue is 4-chlorobutyryl chlorobenzene.
[0036](2) Ring closure reaction: 20L glass reactor equipped with mechanical stirring and thermometer, add 6720g of ethanol under nitrogen protection, add 1440g of sodium hydroxide in batches under stirring, cool down to below 20°C after dissolving and add 4-chlorobutyryl dropwise Chlorobenzene, after dropping, keep warm overnight. The system was distilled under reduced pressure at 60°C until there was no solvent, water and n-hexa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com