Two-photon fluorescence dye, preparation method thereof and application
A technology of two-photon fluorescence and dye, applied in the field of two-photon fluorescent dye and its preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Embodiment 1: the synthesis of compound Lyso-MCO and Lyso-NCO
[0043] 1. Synthesis of Intermediate 1
[0044] Add 2.095g (5mmoL) of 3,6-diiodocarbazole, 0.560g (10mmoL) of KOH, 2.40g (Me(CH 2 CH 2 O) 3 OTs, 7.55mmol) and DMF (30mL), fully stirred and dissolved, and reacted at room temperature for 24 hours, followed by TLC during the reaction; after the reaction was completed, water was added to the reaction solution, then extracted with dichloromethane, and dried over anhydrous sodium sulfate , separated on a silica gel column (eluent: petroleum ether / ethyl acetate=6 / 1, v / v) to obtain 1.695 g of a light yellow solid, namely Intermediate 1, with a yield of 60.0%.
[0045] m.p.72.4-74.6°C. FT-IR (KBr, cm -1 ):2884,1474,1422,1292,1123,798. 1 H NMR (CDCl 3 ,400MHz): δ(ppm)8.29(d,J=1.6Hz,2H),7.70(d,J=1.6Hz,1H),7.68(d,J=1.6Hz,1H),7.22(d,J=1.6Hz,1H) 8.6Hz, 2H), 4.40(t, J=5.6Hz, 2H, CH 2 ), 3.81(t, J=5.6Hz, 2H, CH 2 ),3.52–3.37(m,8H),3.33(s,3H,CH 3 ); 13 C NMR (CDC...
Embodiment 2
[0052] Embodiment 2: Spectral test of two-photon fluorescent dyes Lyso-MCO and Lyso-NCO in DMSO
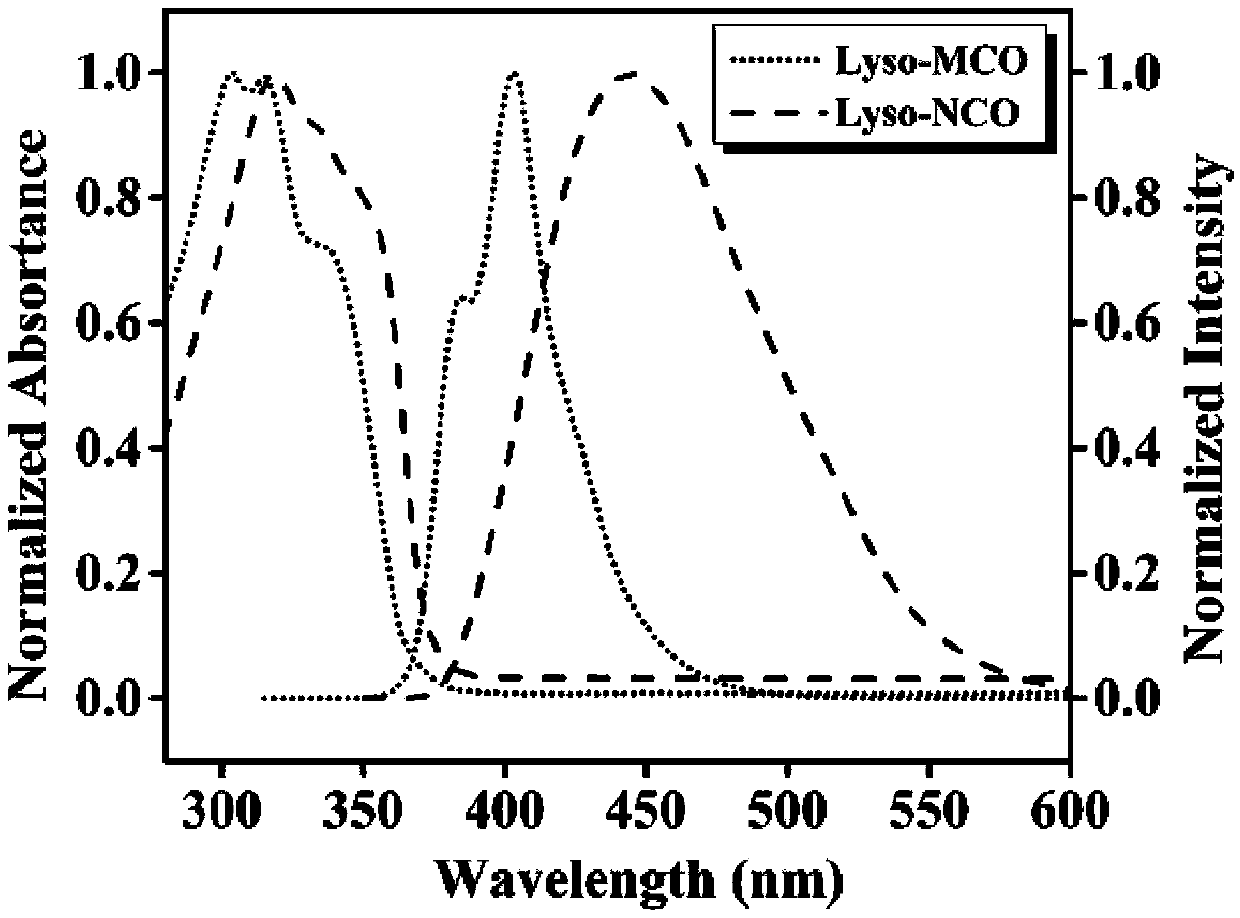
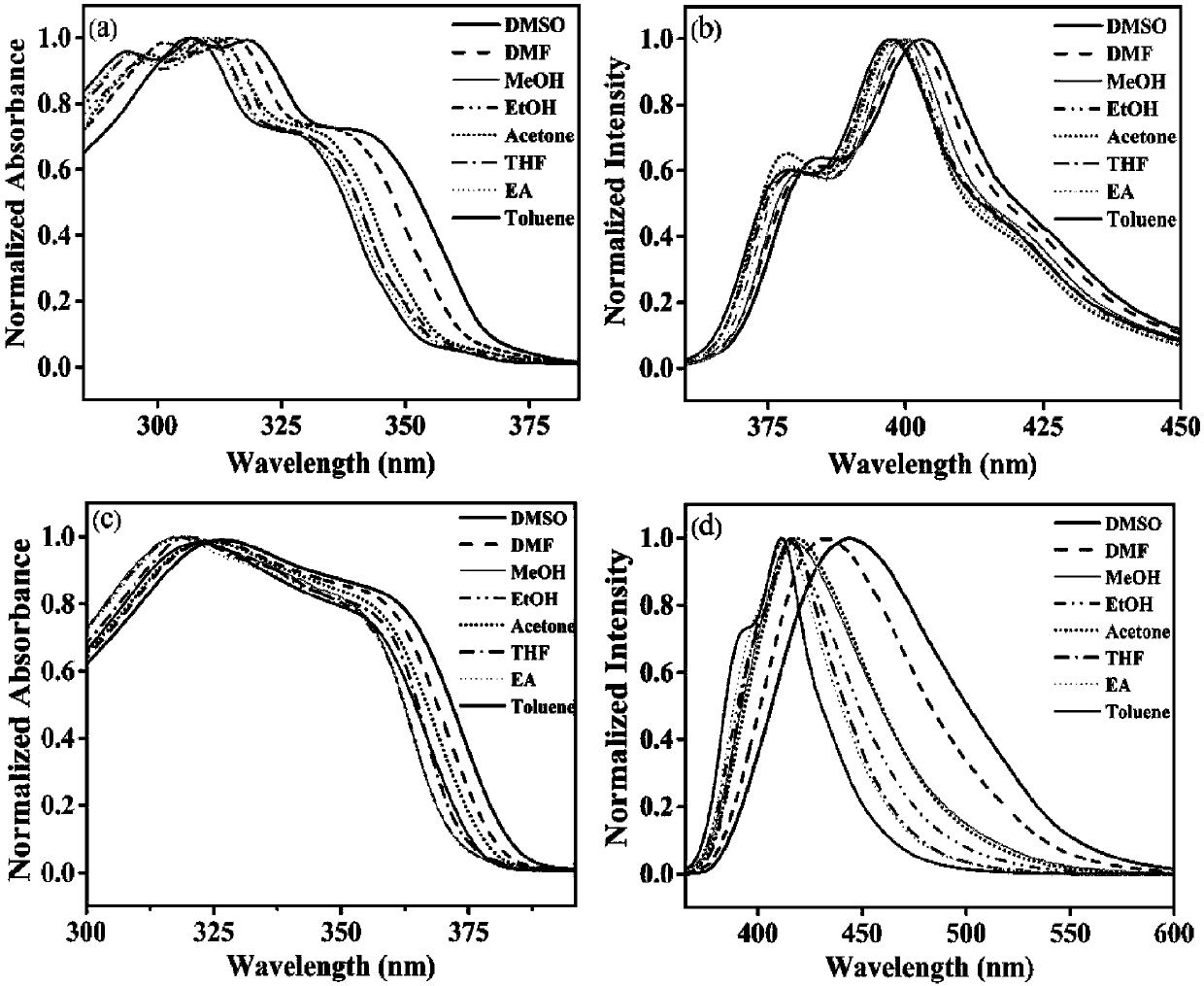
[0053] The two-photon fluorescent dyes Lyso-MCO and Lyso-NCO of the present invention were respectively dissolved in DMSO to prepare a 1mM mother solution. Take 100 μL of the mother solution in a 10mL volumetric flask, and dilute to volume with different solvents to prepare a 10 μM detection reagent. From figure 1 The maximum ultraviolet absorption peaks of the compounds Lyso-MCO and Lyso-NCO are 318 and 328nm, respectively. When Lyso-MCO and Lyso-NCO were excited at 318nm and 328nm respectively, the single-photon fluorescence emission peaks of Lyso-MCO and Lyso-NCO were located at 403nm and 445nm ( figure 1 and Table 1). The compound Lyso-MCO emits blue light, and the compound Lyso-NCO emits blue-green light. Compared with Lyso-MCO, Lyso-NCO has a red shift of about 42nm. This is consistent with the changing law of the ultraviolet absorption spectrum. With the enhancement of t...
Embodiment 3
[0057] Embodiment 3: Two-photon absorption performance test of Lyso-MCO and Lyso-NCO
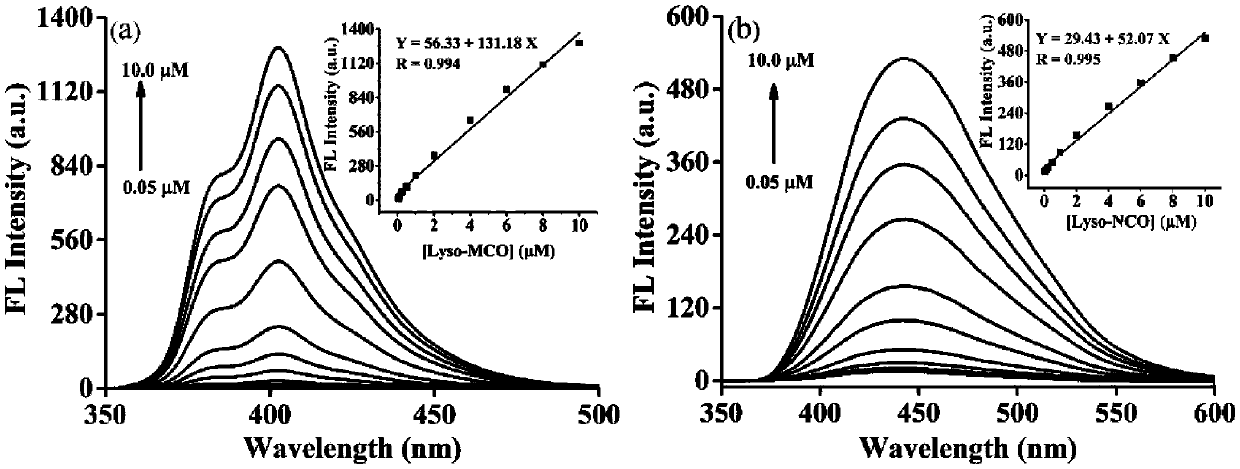
[0058] Using a two-photon induced fluorescence measurement technique, a concentration of 10 -3 The two-photon absorption cross section of the target compound at mol / L, from Figure 5 It can be seen that the maximum effective absorption cross sections (δФ) of Lyso-MCO and Lyso-NCO are 20GM and 43GM respectively, and the maximum two-photon excitation wavelengths are 690nm and 700nm respectively. From Figure 5 It can be seen that the two-photon absorption performance of Lyso-NCO is better than that of Lyso-MCO. When the concentrations of Lyso-MCO and Lyso-NCO are in the range of 0.2-1mM and 0.01-1mM respectively, it can be observed that the two-photon fluorescence emission peak intensity of Lyso-MCO and Lyso-NCO at 418nm and 493nm varies linearly with the concentration enhanced ( Figure 4 ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com