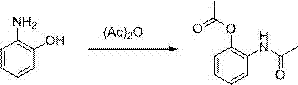Novel preparation method of 3-amino-2-hydroxyacetophenone
A technology of hydroxyacetophenone and a new method, which is applied in the field of preparation of 3-amino-2-hydroxyacetophenone, can solve the problems of long synthetic route and low yield, and achieve novel synthetic route, high purity and low The effect of processing costs on production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] The first step: the preparation of 2-acetamidophenol acetate
[0032] Get 2-aminophenol (109kg), acetic anhydride (224kg), methanol 400kg, concentrated sulfuric acid 10 liters, reflux reaction 4h. After the reaction is finished, the methanol is recovered under reduced pressure, cooled to room temperature, and saturated sodium bicarbonate solution is added to the reaction system Adjusted to pH 9-10, extracted with 400 kg of ethyl acetate, washed with water until neutral, dried over anhydrous sodium sulfate, filtered, and the solvent was recovered under reduced pressure to obtain 191 kg of pale yellow solid with a yield of 99%.
[0033] The second step: the synthesis of 3-amino-2-hydroxyacetophenone
[0034] Take 96 kg of 2-acetamidophenol acetate, dissolve it in 250 kg of N-methylpyrrolidone, add 94 kg of anhydrous titanium tetrachloride, and react at 120 degrees for 3 h. After the reaction, most of the solvent is recovered under reduced pressure, and cooled to room temp...
Embodiment 2
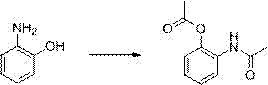
[0036] The first step: the preparation of 2-acetamidophenol acetate
[0037] Get 2-aminophenol (110kg), acetic anhydride (225kg), methanol 410kg, concentrated sulfuric acid 11 liters, backflow reaction 4h. After the reaction, the methanol was recovered under reduced pressure, cooled to room temperature, and saturated sodium bicarbonate solution was added to the reaction system Adjusted to pH 9-10, extracted with 410 kg of ethyl acetate, washed with water until neutral, dried over anhydrous sodium sulfate, filtered, and the solvent was recovered under reduced pressure to obtain 191.5 kg of light yellow solid with a yield of 99.1%.
[0038] The second step: the synthesis of 3-amino-2-hydroxyacetophenone
[0039] Take 95kg of 2-acetamidophenol acetate, dissolve it in 245kg of N-methylpyrrolidone, add 93kg of anhydrous titanium tetrachloride, and react at 120 degrees for 3h. After the reaction, most of the solvent is recovered under reduced pressure and cooled to room temperature....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com