A method for enzymatically synthesizing l-2-aminobutyric acid
A technology of aminobutyric acid and enzymatic synthesis, which can be used in fermentation and other directions, and can solve problems such as low conversion rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Embodiment 1 A method for enzymatically synthesizing 2-aminobutyric acid, the specific steps are as follows:
[0052] 1. Heterologous expression of alanine dehydrogenase alaD and formate dehydrogenase FDH:
[0053] 1. Heterologous expression and enzyme activity determination of alanine dehydrogenase alaD:
[0054] (1) Obtain alaD gene fragment
[0055] Primers were designed according to the alanine dehydrogenase (alaD) gene sequence (Genbank accession number: EF154460, SEQ ID NO.1) and the front and back sequences, and the better upstream primers of alanine dehydrogenase (alaD) obtained after screening The upstream primer is 5'-aaGGATCCatgaagatcggcattccaaaag-3' (the uppercase is the BamHI restriction site, SEQ ID NO.2); the downstream primer is 5'-ttGAATTCtcatccctgcagcaacgaatgaac-3' (the uppercase is the EcoRI restriction site, SEQ ID NO.3). The alaD gene fragment can be efficiently obtained by amplifying with the above-mentioned upstream and downstream primers.
[...
Embodiment 2
[0086] Embodiment 2 A kind of method of enzymatically synthesizing 2-aminobutyric acid
[0087] Except that the following steps are different, all the other steps are the same as in Example 1.
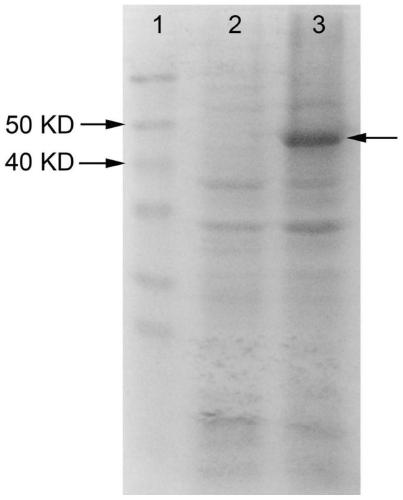
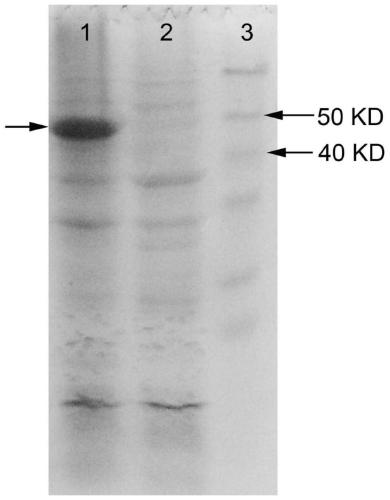
[0088] A genetically engineered bacterium co-expressing alaD and FDH was constructed, and a bacterium co-expressing FDH and alaD was obtained through fermentation. The construction of the plasmids pET32a-alaD and pET28a-FDH is the same as in Example 1, and the plasmids pET32a-alaD and pET28a-FDH are transferred into the same strain E.coli BL21(DE3) to obtain the strain BL21-alaD / FDH co-expressing alaD and FDH , recombinant protein induction of co-expression strains see Figure 5 (Swimming lane 3 arrow shows the co-expressed induced protein, induction 2 is the empty vector negative control, induction 1 is the protein molecular weight standard).
[0089] (1) The fermentation of the co-expression bacteria was the same as in Example 1.
[0090] (2) Add the following pharmaceutical prepa...
Embodiment 3
[0091] Embodiment 3 A kind of method of enzymatically synthesizing 2-aminobutyric acid
[0092] In addition to the following steps "Cultivate the strain BL21-alaD in the fermentation medium, add 0.1% lactose to induce expression at 30°C for 16 hours, collect the cells by centrifugation to obtain the expression cells of alaD, and store the expressed cells at -20°C ; Culture the bacterial strain BL21-FDH in the fermentation medium, add 0.1% lactose to induce expression at 30°C for 16 hours, collect the cells by centrifugation to obtain the FDH expressing cells, and store the expressing cells at -20°C for induction temperature. Be that 30 ℃ is changed into 25 ℃, all the other are with embodiment 1.
[0093] Then obtain the expression cells of alaD and FDH by the method in the first step, add the following medicine preparation catalyst system successively in the 250ml Erlenmeyer flask: 0.5mol of 2-ketobutyric acid, 0.5mol of ammonium formate, wet cells of formate dehydrogenase 20...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com