Pelteobagrus fulvidraco beta defensin gene, and beta defensin antibacterial peptide and application thereof
A technology of β-defensins and antimicrobial peptides, applied in the field of molecular biology, can solve the problems of recombinant protein antibacterial experiments, different antibacterial effects of recombinant proteins, and in-depth research, and achieve good broad-spectrum antibacterial activity in vitro Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
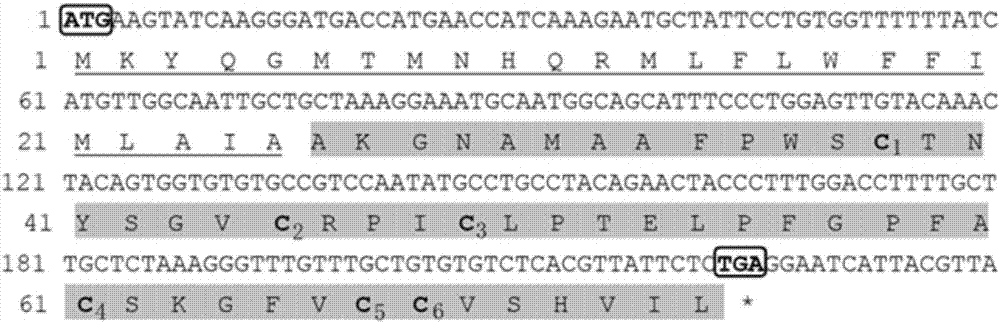
[0051] Example 1 Cloning of the open reading frame (ORF) fragment of the β-defensin gene of yellow catfish.
[0052] 1. Cloning of the open reading frame (ORF) fragment of the β-defensin gene of yellow catfish
[0053] According to the partial transcriptome data of the yellow catfish in our laboratory, the predicted sequence of the yellow catfish β-defensin gene was retrieved, and Primer 5.0 was used to design the primer Pf_ΒD-F1 / R1 for amplifying the ORF region of the β-defensin:
[0054] Pf_ΒD-F1: ATGAAGTATCAAGGGATGACCAT
[0055] Pf_ΒD-R1: TCAGAGAATAACGTGAGACACAC
[0056] The PCR reaction system is as shown in Table 1-1:
[0057] Table 1-1 PCR reaction system
[0058]
[0059] PCR reaction conditions: pre-denaturation at 95°C for 3 min; denaturation at 95°C for 30 s, annealing at 58°C for 30 s, extension at 72°C for 30 s, a total of 35 cycles; extension at 72°C for 10 min; 16°C for 5 min. After the reaction, 20 μL of the PCR product was added to 4 μL of 6×loading buff...
Embodiment 2
[0089] Example 2 The cloning of the core coding region of the yellow catfish β-defensin gene (with the signal peptide removed from the ORF) and the construction of the prokaryotic recombinant expression plasmid.
[0090] According to the characteristics of the restriction site on the prokaryotic expression plasmid PET-32a, a pair of specific primers Pf_BD-F2 / R2 with added BamHI and EcoR I restriction sites were specially designed:
[0091] Pf_ΒD-F2: CGC GGATCC GCTAAAGGAAATGCAATGGC
[0092] Pf_ΒD-R2: CCG GAATTC TCAGAGAATAACGTGAGACACAC
[0093]The underlined parts of the primer sequences are the restriction sites of BamH I and EcoR I, respectively, and the bold parts (CGC and CCG) at the 5' end of the restriction sites are protective bases. Using PCR amplification technology, the ORF fragment cloned in Experiment 1 was used as the cDNA template, and the reaction program was (first, pre-denaturation at 95°C for 5 minutes; then, denaturation at 95°C for 30 seconds, annealing a...
Embodiment 3
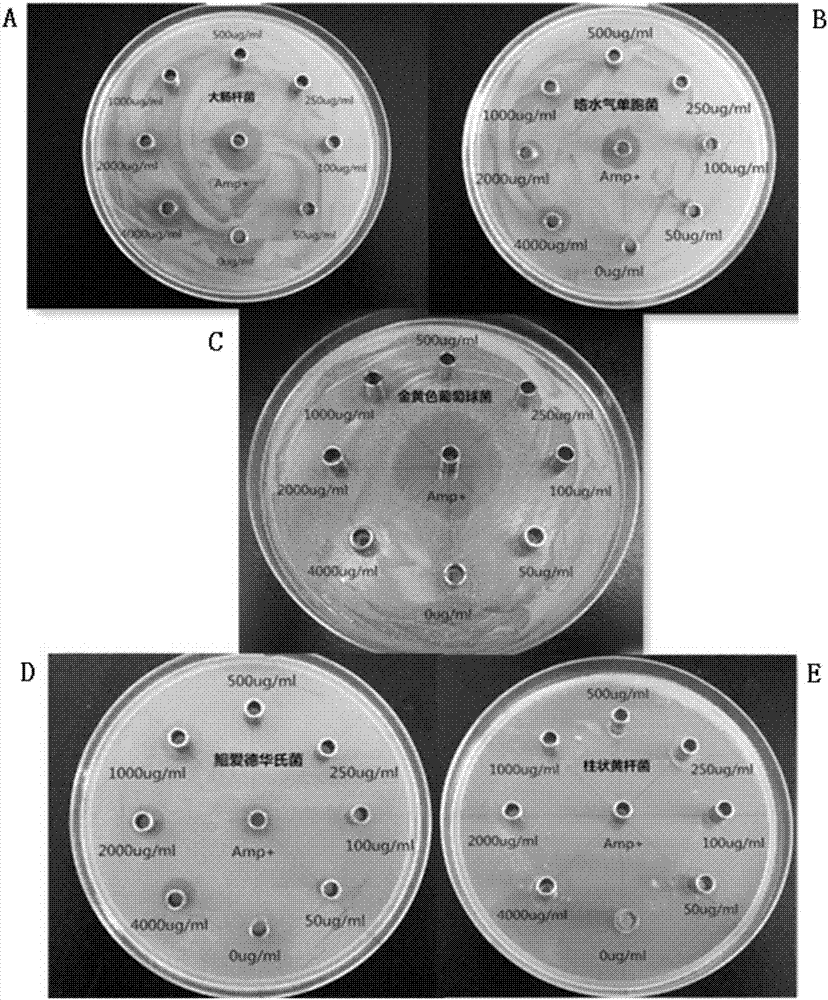
[0094] Example 3: Induction and expression of prokaryotic recombinant expression vectors in Escherichia coli.
[0095] The constructed recombinant expression vector PET-32a-Pf_BD was introduced into Escherichia coli BL21 (DE3) competent cells, and the positive clones were picked on the plate and inoculated in 10 mL of LB liquid medium containing ampicillin, cultivated overnight at 37 ° C, 200 rpm, and added An equal volume of 30% glycerol was stored at -35°C for later use.
[0096] Exploration of the best induction expression conditions:
[0097] (1) Induction of expression at different times: Take 1 mL of spare bacteria and add it to 9 mL of LB liquid medium, cultivate to OD 600 0.6 to 0.8, then take 1mL of the bacterial solution and divide it into 2mL preparation tubes, a total of 7 tubes, at the same time take 1mL of the PET-32a empty plasmid bacterial solution under the same conditions as a control, and add 8 tubes of bacterial solution with a final concentration of 1mM ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com