Preparation method of methylene bridged cucurbituril
A technology of methylene bridge uride and methylene bridge is applied in the field of preparation of methylene bridge cucurbituril, which can solve the problem of poor stability of methylene bridge cucurbituril, inability to separate, and yield of methylene bridge cucurbituril. Low problems, to achieve the effects of low cost, reduced preparation cost, and simplified reaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
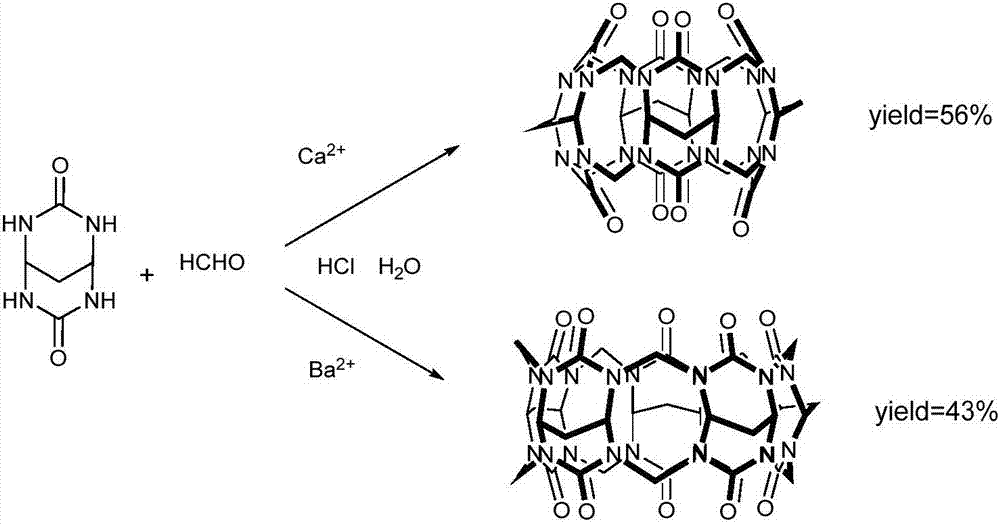
[0032] Put methylene glucoside urea (10g, 64mmol), paraformaldehyde (5g, 167mmol) and calcium chloride (0.5g, 4.5mmol) in a reaction flask, add 5mL of water and 15mL of hydrochloric acid, stir and heat up to 100°C , reacted for 8 hours, a white solid was precipitated, and filtered to obtain the product methylene cucucurbit[4]urea, which was dried to obtain 3.61 g with a yield of 31.3%.
[0033] The chemical shift value of the product in the NMR spectrum is: 1 H NMR (400MHz,D 2 O, ppm) δ: 6.36 (d, 8H, J=16), 5.23 (s, 8H), 4.19 (d, 8H, J=16), 2.32 (s, 8H).
[0034] The molecular weight of the compound obtained by high-resolution mass spectrometry is: m / z calculate for [C 28 h 32 N 16 o 8 +H + ]:721.26,found:721.26.
Embodiment 2
[0036] Place methylene urea (10g, 64mmol), paraformaldehyde (5g, 167mmol) and calcium chloride (1g, 9mmol) in a reaction flask, add 5mL of water and 15mL of hydrochloric acid, stir and heat up to 100°C, react After 8 hours, a white solid was precipitated, filtered to obtain the product methylene cucurbit[4]urea, and after drying, 6.22g was obtained after drying, with a yield of 54.08%
[0037] The chemical shift value of the product in the NMR spectrum is: 1 H NMR (400MHz,D 2 O, ppm) δ: 6.36 (d, 8H, J=16), 5.23 (s, 8H), 4.19 (d, 8H, J=16), 2.32 (s, 8H).
[0038] The molecular weight of the compound obtained by high-resolution mass spectrometry is: m / z calculate for [C 28 h 32 N 16 o 8 +H + ]:721.26,found:721.26.
Embodiment 3
[0040] Place methylene glucoside urea (10g, 64mmol), paraformaldehyde (5g, 167mmol) and calcium nitrate (1.5g, 9mmol) in a reaction flask, add 5mL of water and 15mL of hydrochloric acid, stir and heat up to 100°C, and react 8h, a white solid was precipitated, filtered to obtain the product methylene cucurbit[4] urea, and after drying, 3.22g was obtained, with a yield of 28%
[0041] The chemical shift value of the product in the NMR spectrum is: 1 H NMR (400MHz,D 2 O, ppm) δ: 6.36 (d, 8H, J=16), 5.23 (s, 8H), 4.19 (d, 8H, J=16), 2.32 (s, 8H).
[0042] The molecular weight of the compound obtained by high-resolution mass spectrometry is: m / z calculate for [C 28 h 32 N 16 o 8 +H + ]:721.26,found:721.26.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com