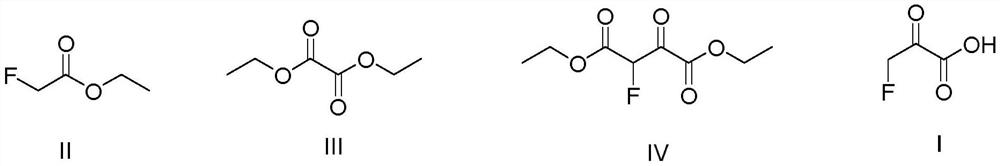
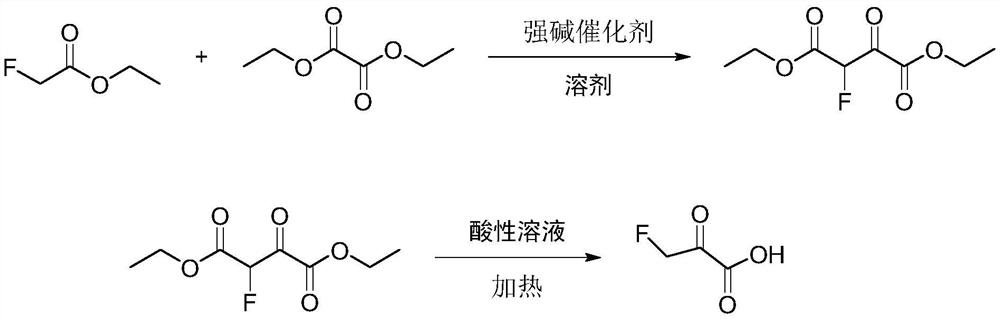
A kind of preparation method of 3-fluoropyruvate
A technology of fluoropyruvic acid and ethyl acetate, which is applied in the field of preparation of 3-fluoropyruvic acid, can solve the problems of low yield, high production cost, long reaction steps and the like, and achieves simple process conditions and low production cost. , the effect of easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Add 600ml tetrahydrofuran, NaH solid (24.0g, 1.00mol) and diethyl oxalate (140.0g, 0.95mol) into a 1L four-necked round bottom flask equipped with mechanical stirring, slowly Ethyl fluoroacetate (100.0 g, 0.94 mol) was slowly added dropwise, and the reaction was carried out at 70° C. for 6 h after the addition was completed. Add 400ml of toluene, 500.0g of ice, and 88ml of concentrated hydrochloric acid (mass concentration 36-38%) into a 2L beaker, cool down to 0°C, and slowly add the reaction solution dropwise to the 2L beaker to quench excess NaH. Separation, the aqueous layer was extracted with toluene (100ml x 3), the organic phases were combined, after reclaiming the toluene solvent, rectification, and the gas phase temperature of 100-101 ° C was collected to obtain diethyl 2-fluoro-3-oxosuccinate ( 168.9g, 0.82mol), the gas chromatography detection purity is 99.9%, and the yield is 86.9%.
[0026] Gas chromatography testing conditions: Chromatographic column: cap...
Embodiment 2
[0030] Add 1000ml tetrahydrofuran, sodium methoxide solid (80.0g, 2.00mol), diethyl oxalate (280.0g, 1.9mol) in a 2L four-necked round bottom flask equipped with mechanical stirring, and use a constant pressure dropping funnel at room temperature Ethyl fluoroacetate (200.0 g, 1.88 mol) was slowly added dropwise, and the reaction was carried out at 65° C. for 6 h after the addition was completed. Add 400ml of toluene, 1000.0g of ice, and 180ml of concentrated hydrochloric acid (mass concentration 36-38%) into a 5L beaker. Excess sodium hydride. Separation, the aqueous layer was extracted with toluene (100ml x 3), the organic phases were combined, after reclaiming the toluene solvent, rectification, and the gas phase temperature of 100-101 ° C was collected to obtain diethyl 2-fluoro-3-oxosuccinate ( 323.4g (1.57mol), gas chromatography detection purity: 99.9%, yield: 83.5%.
[0031] Add diethyl 2-fluoro-3-oxosuccinate (323.4 g, 1.57 mol) and 200 ml of concentrated hydrochlori...
Embodiment 3
[0033] Add 1000ml tetrahydrofuran, sodium ethoxide solid (136.0g, 2.00mol) and diethyl oxalate (328.0g, 2.28mol) in a 2L four-neck round-bottomed flask equipped with mechanical stirring. Ethyl fluoroacetate (200.0 g, 1.88 mol) was slowly added dropwise, and the reaction was carried out at 60° C. for 6 h after the addition was completed. Add 400ml of toluene, 1000.0g of ice, and 180ml of concentrated hydrochloric acid (mass concentration 36-38%) into a 5L beaker. Excess sodium ethoxide. Separation, the aqueous layer was extracted with toluene (100ml x 3), the organic phases were combined, after reclaiming the toluene solvent, rectification, and the gas phase temperature of 100-101 ° C was collected to obtain diethyl 2-fluoro-3-oxosuccinate ( 327.6g (1.59mol), the gas chromatography detection purity is 99.9%, and the yield is 84.6%.
[0034] Add diethyl 2-fluoro-3-oxosuccinate (327.6g, 1.59mol) into a 1L four-neck flask equipped with mechanical stirring, 200ml of concentrated ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com