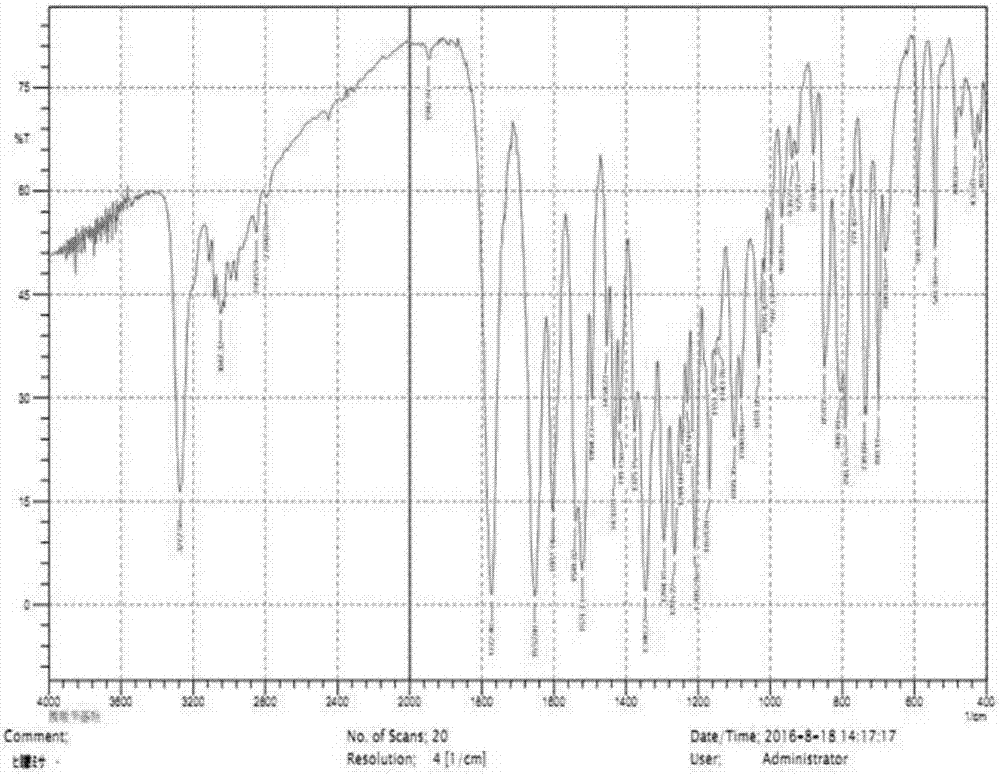
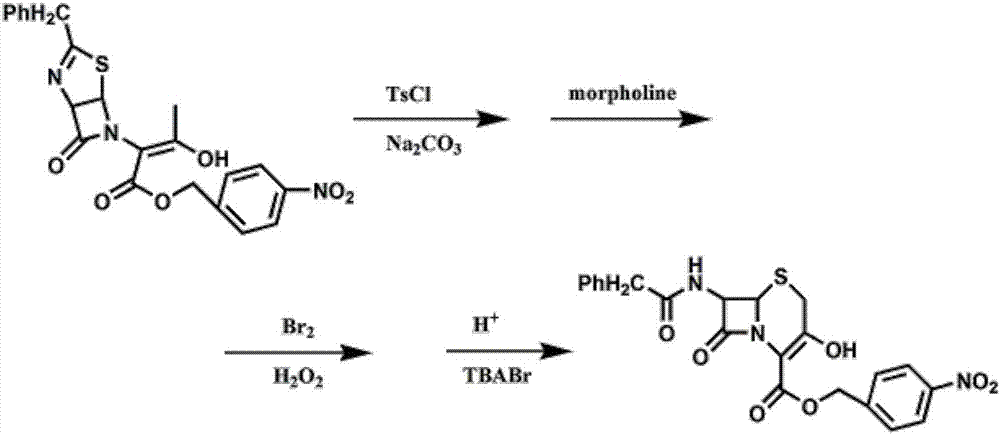
Method for preparing 7-phenylacetylamino-3-hydroxyl-3-cyclo-4-p-nitrobenzyl cephalosporin carboxylate
A technology of phenylacetamido and nitrobenzyl ester, applied in directions such as organic chemistry, can solve the problems of production restriction of various cephalosporins, easy environmental pollution, serious environmental pollution, etc., and achieves shortened reaction time, low cost, Simple to use effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Preparation of p-nitrobenzyl 7-phenylacetamido-3-hydroxy-3-cephalosporin-4-carboxylate.
[0029] Add 150mL of dichloromethane (as an organic solvent) and 22.7g of thiazoline enol ester to a 500mL four-neck flask, cool down to -5°C, add 10.5g of p-toluenesulfonyl chloride, add 10.6g of sodium carbonate, keep warm for 2h, TLC Monitor, add 4.8g of morpholine dropwise, finish dropping in 10 minutes, stir for 30min, monitor by TLC, then add 4.8gBr2 dropwise, finish dropping in 10 minutes, start adding 3.4g of 30% hydrogen peroxide dropwise after 15min, finish dropping in 20 minutes, TLC Monitor, use 8% sulfuric acid aqueous solution to adjust pH=0.8~1.0, add catalyst tetrabutylammonium bromide (TBABr) 1.2g, heat up to 30 ℃ and react for 3 hours, TLC monitors, add water 32mL, fully stir for 10 minutes, divide liquid, the organic phase was adjusted to pH=7 with 2% sodium bicarbonate, and the above organic phase was washed twice with 100 mL of water, and all the above water pha...
Embodiment 2
[0031] Preparation of p-nitrobenzyl 7-phenylacetamido-3-hydroxy-3-cephalosporin-4-carboxylate.
[0032] Add 150mL of dichloromethane and 22.7g of thiazoline enol ester to a 500mL four-neck flask, cool down to -5°C, add 10.5g of p-toluenesulfonyl chloride, add 10g of sodium carbonate, keep warm for 2h, monitor by TLC, and then dropwise add molybdenum 4.8g of morphine, dropped in 10 minutes, stirred for 30min, monitored by TLC, then added dropwise 4.8gBr2, added dropwise in 10 minutes, after 15min, started to add 3.4g of 30% hydrogen peroxide, dropped in 20 minutes, monitored by TLC, with 8% Sulfuric acid aqueous solution to adjust pH=0.8~1.0, add 1.2g of catalyst tetrabutylammonium bromide (TBABr), heat up to 30°C and react for 3 hours, monitor by TLC, add 32mL of water, fully stir for 10 minutes, separate liquid, use for organic phase Adjust the pH to 7 with 2% sodium bicarbonate, wash the above organic phase twice with 100 mL of water, combine all the above water phases, extr...
Embodiment 3
[0034] Preparation of p-nitrobenzyl 7-phenylacetamido-3-hydroxy-3-cephalosporin-4-carboxylate.
[0035] Add 150mL of dichloromethane and 22.7g of thiazoline enol ester to a 500mL four-neck flask, cool down to -5°C, add 10.5g of p-toluenesulfonyl chloride, add 10.6g of sodium carbonate, keep warm for 2h, monitor by TLC, and then add dropwise Morpholine 6.5g, dripped in 10 minutes, stirred for 30min, monitored by TLC, then added dropwise 4.8gBr2, added dropwise in 10 minutes, after 15min, began to drop 3.4g of 30% hydrogen peroxide, dripped in 20 minutes, monitored by TLC, used 8 % sulfuric acid aqueous solution to adjust the pH=0.8~1.0, add catalyst tetrabutylammonium bromide (TBABr) 1.2g, heat up to 30°C and react for 3 hours, monitor by TLC, add water 32mL, fully stir for 10 minutes, separate liquid, organic phase Use 2% sodium bicarbonate to adjust pH=7, wash the above organic phase with 100mL water twice, combine all the above water phases, extract the combined water phase ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com