Preparation method for carbon-loaded iron nitride compound sodium ion battery negative electrode material doped with nitrogen
A technology of sodium ion battery and negative electrode material, applied in the field of electrochemistry, can solve the problems of poor conductivity, limited wide application, low capacity, etc., and achieve the effects of easy operation, low cost and simple method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] 1) Dissolve urea, polyvinylpyrrolidone PVP and ferric ammonium oxalate in deionized water at a mass ratio of 40:1:10, stir evenly, add 6g of urea to every 40mL of deionized water, and freeze-dry it for 12 hours, the product is Gel, denoted as A;
[0031] 2) Put A in a vacuum tube furnace pyrolysis reaction, Ar gas is the protective gas, the pyrolysis temperature is 400°C, the reaction time is 3h, the heating method is gradient heating, and the temperature is raised to 200°C at a heating rate of 5°C / min. ℃, keep warm for 0.5h, then rise to the final pyrolysis temperature at 10℃ / min for reaction, and record the obtained product as B;
[0032] 3) The above-mentioned preparation B product was washed three times with deionized water and ethanol respectively, and unstable products were removed to obtain a nitrogen-doped carbon-supported iron nitride composite sodium-ion battery negative electrode material.
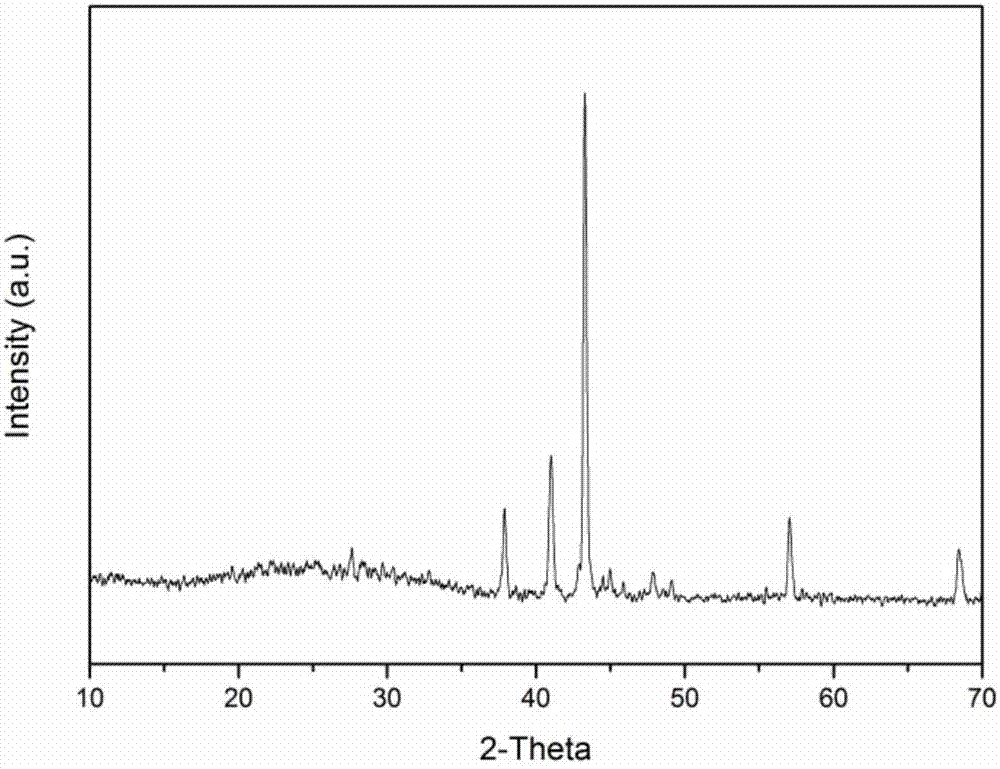
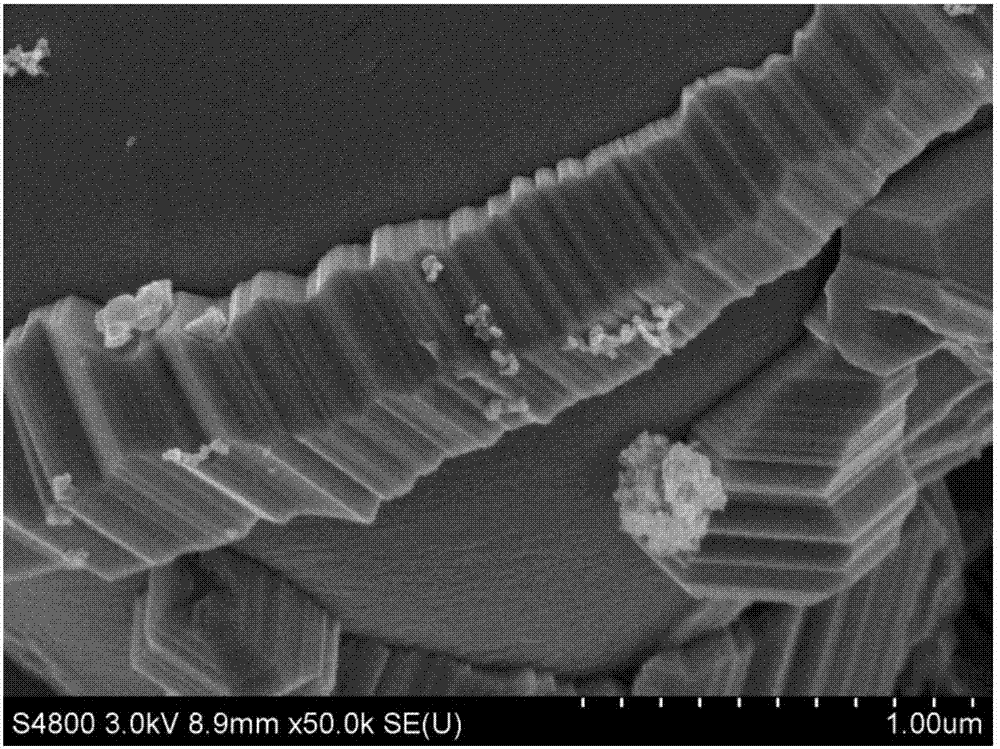
[0033] see figure 1 , the resulting product particles were analyze...
Embodiment 2
[0037] 1) Dissolve melamine, chitosan and ferric acetate in deionized water at a mass ratio of 40:1:40, stir evenly, add 6g of melamine per 40mL of deionized water, and then freeze-dry it for 12 hours, and the product is a gel shape, denoted as A;
[0038] 2) Put A in a vacuum tube furnace pyrolysis reaction, Ar gas is the protective gas, the pyrolysis temperature is 800°C, the reaction time is 1h, the heating method is gradient heating, and the temperature is raised to 300°C at a heating rate of 10°C / min , keep warm for 0.5h, then rise to the final pyrolysis temperature at 15°C / min for reaction, and record the obtained product as B;
[0039] 3) The above-mentioned preparation B product was washed three times with deionized water and ethanol respectively, and unstable products were removed to obtain a nitrogen-doped carbon-supported iron nitride composite sodium-ion battery negative electrode material.
Embodiment 3
[0041] 1) Dissolve melamine, chitosan and ferric acetate in deionized water at a mass ratio of 40:1:20, stir evenly, add 6g of urea to every 40mL of deionized water, and freeze-dry it for 12 hours, the product is gel shape, denoted as A;
[0042] 2) Put A in a vacuum tube furnace pyrolysis reaction, Ar gas is the protective gas, the pyrolysis temperature is 600°C, the reaction time is 2h, the heating method is gradient heating, and the temperature is raised to 300°C at a heating rate of 5°C / min , keep warm for 0.5h, then rise to the final pyrolysis temperature at 10°C / min for reaction, and record the obtained product as B;
[0043]3) The above-mentioned preparation B product was washed three times with 80° C. deionized water and ethanol respectively to remove unstable products, that is, a nitrogen-doped carbon-supported iron nitride composite sodium-ion battery negative electrode material was obtained.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com