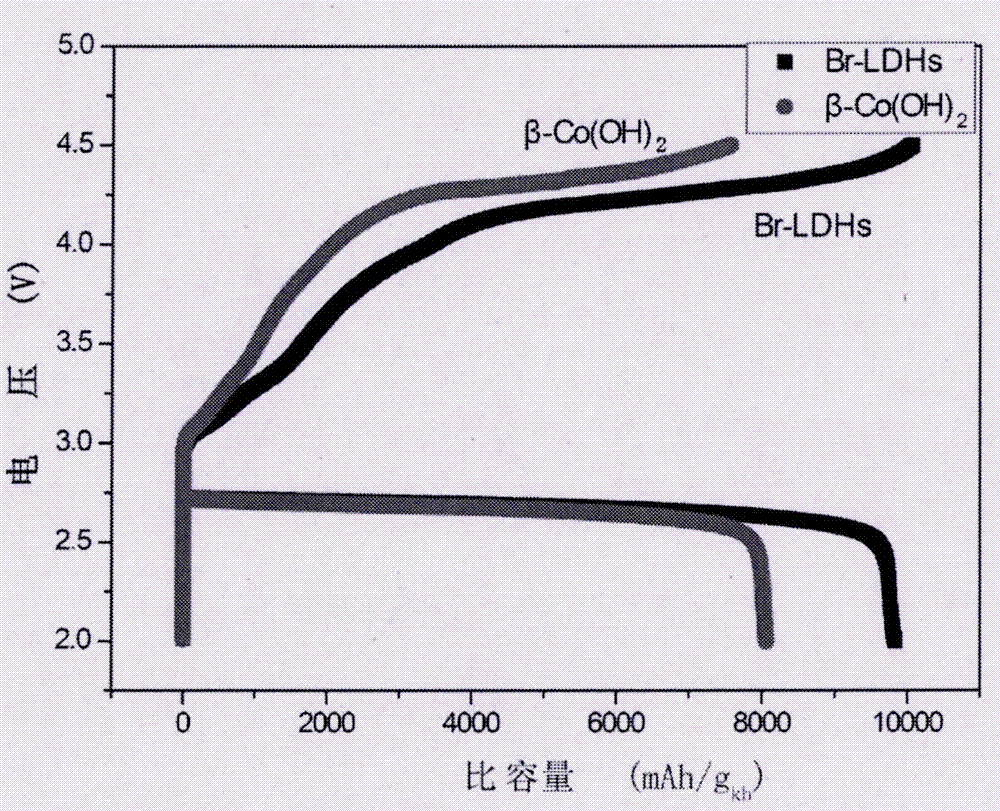
Application of bromide-intercalated lamellar Co2+/Co3+ hydroxide in lithium-air batteries
A lithium-air battery, hydroxide technology, applied in cobalt oxide/cobalt hydroxide, fuel cell-type half-cell and secondary battery-type half-cell, battery electrodes, etc., can solve the problem of high price, poor catalytic performance, It is difficult to popularize and other problems to achieve the effect of low price, good catalytic activity, and improved cycle stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Example 1 Preparation of β-Co(OH) 2
[0043] First, take 1000ml of deionized water and add it to a round-bottomed flask. Under nitrogen protection, heat and boil. After cooling to room temperature naturally, take 1.2g of cobalt chloride hexahydrate and 12.6g of hexamethylenetetramine (HMT). In the flask, feed nitrogen gas and supplemented by magnetic stirring, heat to 100°C for three hours, then perform centrifugal filtration, wash the obtained powder twice with deionized water and ethanol, and place in a vacuum oven at a temperature of 80°C 12h. After drying, a pinkish-purple solid powder is obtained, that is, β-Co(OH) 2 .
Embodiment 2
[0044] Example 2 Preparation of bromine-intercalated layered Co 2+ / Co 3+ Hydroxide (hereinafter referred to as Br-LDHs)
[0045] The 0.25gβ-Co(OH) synthesized by embodiment 1 2 The sample was placed in 200ml of carbon tetrachloride solution and mechanically stirred until it was evenly dispersed, then 2ml of liquid bromine was added, and after stirring at room temperature for 12 hours, it was suction filtered and washed with carbon tetrachloride until the carbon tetrachloride was clear. At this time, the product The liquid bromine in the solution has been removed, and then placed in an oven at 80°C for 12 hours, and dried to obtain brown-gray Br-LDHs.
[0046] Characterization Examples
Embodiment 3
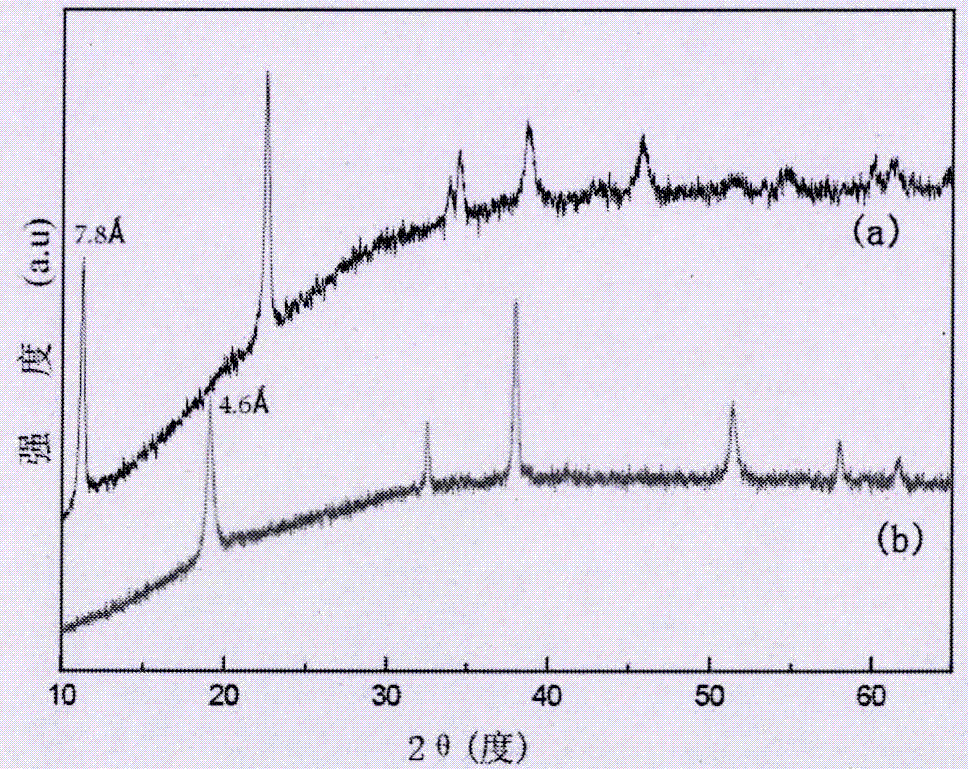
[0047] Embodiment 3 XRD analysis
[0048] Adopt the X-ray powder diffractometer (model: X Pert PRO MPD) that Holland Phillips company produces to the β-Co(OH) that embodiment 1 prepares 2 1. The Br-LDHs prepared in Example 2 were characterized by XRD, the radioactive source was Cu-Ka, the measurement step was 0.017°, and the scan time was 10 seconds / step. The result is as figure 1 shown;
[0049] from figure 1 It can be seen from the figure (b) that the synthesized precursor β-Co(OH) 2 The diffraction peaks are consistent with the standard card (JCPDS30-0443); figure 1 It can be seen from the figure (a) in the figure that the β-Co(OH) treated with liquid bromine 2 (ie Br-LDHs), the interplanar spacing of the (001) crystal plane increases from 4.6A to 7.8A, which is closely related to the intercalation of bromide ions into the interlayer, and part of the divalent cobalt is oxidized into trivalent cobalt.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Interplanar spacing | aaaaa | aaaaa |
| Diameter size | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com