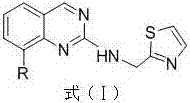
Drug for treating acquired immunodeficiency syndrome (AIDS) and preparation method thereof
A technology for AIDS and medicine, applied in the field of medicine for the treatment of AIDS and its preparation, can solve the problems of unsatisfactory cancer cell inhibitory activity and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
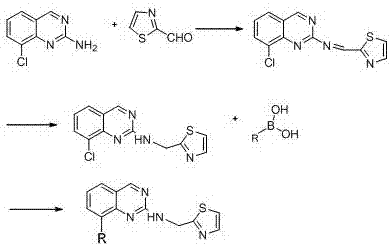
[0021] Example 1: Synthesis of intermediate N-(thiazole-2-methylene)-8-chloroquinazolin-2-amine
[0022]
[0023] Dissolve 8-chloroquinazolin-2-amine (10 mmol) and 2-thiazole formaldehyde (11 mmol) in 30 ml of methanol / tetrahydrofuran (2:1) mixed solvent, reflux for 1 hour, then cool down to room temperature, and add to the system Add water (50 ml), extract with 50 ml of dichloromethane, dry the organic phase over anhydrous sodium sulfate, evaporate the solvent under reduced pressure, recrystallize with acetone and ethanol (1:4), filter, and dry under vacuum at 50°C. 2.6 g of dark yellow N-(thiazole-2-methylene)-8-chloroquinazolin-2-amine solid was obtained, with a yield of 95%. 1 H-NMR (400 MHz, CDCl 3 ) δ: 7.41-7.48(m,2H), 7.80-7.84(m,2H), 8.12(d,1H), 8.66(s,1H), 9.74(s,1H).
Embodiment 2
[0024] Example 2: Synthesis of intermediate N-(thiazole-2-methyl)-8-chloroquinazoline-2-secondary amine
[0025]
[0026] Dissolve N-(thiazole-2-methylene)-8-chloroquinazolin-2-amine (10 mmol) in 40 ml of methanol, then add 5 ml of glacial acetic acid, cool to 0-5°C, and then add 1.2 gram of sodium borohydride, stirred at low temperature for 2 hours, raised to room temperature and continued to stir for half an hour, then added 100 ml of water to the system, stirred for 1 hour, filtered, and vacuum-dried at 50°C to obtain 2.54 g of brown-yellow N-(thiazole-2 -methyl)-8-chloroquinazoline-2-secondary amine solid powder, yield 92%. 1 H-NMR (400 MHz, CDCl 3 ) δ: 3.29(s,1H), 4.65(s,2H), 7.20(d,1H), 7.48(t,1H), 7.65(d,1H), 7.79(m,1H), 8.12(m,1H ), 9.43(s,1H).
Embodiment 3
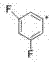
[0027] Example 3: Synthesis of N-(thiazole-2-methyl)-8-phenylquinazoline-2-secondary amine
[0028]
[0029] Dissolve N-(thiazole-2-methyl)-8-chloroquinazoline-2-secondary amine (10 mmol) in 30 ml of nitrogen dimethylformamide, blow the system with argon for 20 minutes, and evacuate the system Then add tetrakis triphenylphosphine palladium (1 mmol), heat up to 60 ° C, continue to stir for half an hour, add phenylboronic acid (12 mmol) to it, then add 10 ml of sodium carbonate (1 g) aqueous solution, heat up Stir at 90°C for 5 hours, keep argon flowing during the whole process, then lower the temperature, evaporate the solvent under reduced pressure, and the solid passes through the chromatographic column quickly to obtain 2.9 g of off-white solid, which is N-(thiazole-2-methyl) -8-phenylquinazoline-2-secondary amine, yield 91%. 1 H-NMR (400 MHz, CDCl 3 ) δ: 3.18(s,1H), 4.65(s,2H), 7.18-7.21(m,5H), 7.41(m,1H),7.65-7.73(m,2H), 7.99-8.04(m,2H) , 9.43(s,1H). 13 C-NMR (75 MH...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com