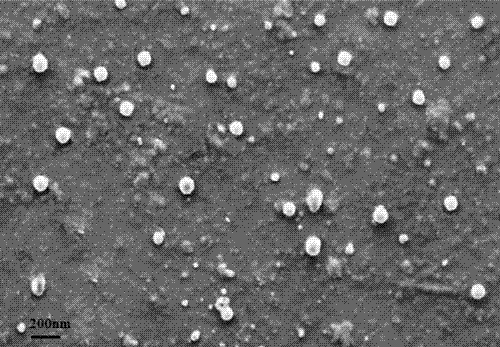
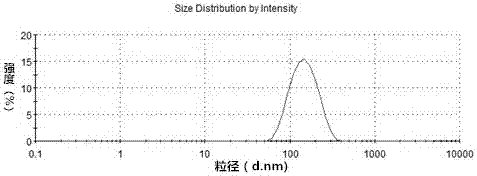
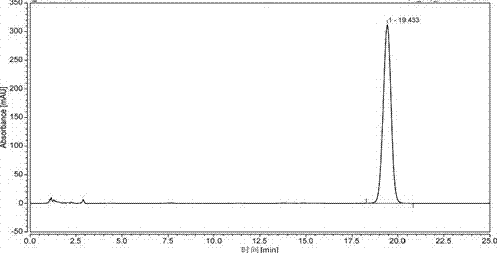
Tanshinone IIAPEG-PLGA-PEG (Tanshinone Polyethylene Glycol-Poly(Lactic-Co-Glycolic Acid)-Polyethylene Glycol) nanoparticles and preparation method thereof
A technology of tanshinone and nanoparticles, which is applied in the field of medicine, can solve the problems of reaching the target site and difficult drugs, and achieve the effects of changing distribution, avoiding side effects, and prolonging the half-life of blood
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Example 1 Preparation of Tanshinone IIA PEG-PLGA-PEG Nanoparticles Encapsulated
[0030] (1) 2 mg of tanshinone IIA and 30 mg of PEG-PLGA-PEG were ultrasonically dissolved in a mixed solvent of 2 ml of acetone and dichloromethane (acetone:dichloromethane = 1:9) as the oil phase, and the prepared mass fraction was 0.5% The poloxamer F188 solution is used as the aqueous phase;
[0031] (2) Under the condition of stirring in an ice-water bath, use a syringe to draw 30 μL of the inner aqueous phase in step (1) and drop it into the organic phase at a speed of 10-30 drops / min, sonicate for 5 s, intermittently for 5 s, and continuously sonicate for 10 min the formation of colostrum;
[0032] (3) Under the condition of stirring in an ice-water bath, draw the colostrum in step (2) with a syringe, and add dropwise to 20 ml 0.5% polo at a rate of 10-30 drops / min. In Sharm F188 solution (the ratio of colostrum to double milk is 1:10), ultrasonication was performed for 5 s, interm...
Embodiment 2
[0034] Example 2 Preparation of Tanshinone IIA PEG-PLGA-PEG Nanoparticles Encapsulated
[0035] (1) Sonicate 4 mg of tanshinone IIA and 30 mg of PEG-PLGA-PEG in a mixed solvent of 2 ml of acetone and methylene chloride (acetone: methylene chloride = 1:8) as the oil phase, and prepare a mass fraction of 0.5% The Tween-80 solution is used as the water phase;
[0036] (2) Under the condition of stirring in an ice-water bath, use a syringe to draw 30 μL of the inner aqueous phase in step (1) and drop it into the organic phase at a speed of 10-30 drops / min, sonicate for 5 s, intermittently for 5 s, and continuously sonicate for 10 min the formation of colostrum;
[0037] (3) Under the condition of stirring in an ice-water bath, draw the colostrum in step (2) with a syringe, and add it dropwise to 20 ml 0.5% Tween at a rate of 10-30 drops / min. -80 solution, ultrasonic 5 s intermittent 5 s, continuous ultrasonic 20 min to form double emulsion;
[0038] (4) Stir at room temperature...
Embodiment 3
[0039] Example 3 Preparation of Tanshinone IIA PEG-PLGA-PEG Nanoparticles Encapsulated
[0040] (1) Sonicate 8 mg of tanshinone IIA and 30 mg of PEG-PLGA-PEG in a mixed solvent of 2 ml of acetone and dichloromethane (acetone:dichloromethane = 1:8) as the oil phase, and prepare a mass fraction of 0.5% The PVA solution is used as the inner water phase;
[0041] (2) Under the condition of stirring in an ice-water bath, use a syringe to draw 30 μL of the inner aqueous phase in step (1) and drop it into the organic phase at a speed of 10-30 drops / min, ultrasonic for 5 s, intermittent for 5 s, and continuous ultrasonic for 10 min the formation of colostrum;
[0042] (3) Under the condition of stirring in an ice-water bath, draw the colostrum in step (2) with a syringe, and add it dropwise to 20 ml of 0.5% PVA solution at a speed of 10-30 drops / minute under the liquid surface Medium (the ratio of colostrum to double milk is 1:10), ultrasound for 5 s, intermittent 5 s, continuous ul...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com