Fatigue relieving composition and preparation method and application thereof
A technology for relieving fatigue and composition, which is applied in drug combinations, medical preparations containing active ingredients, and pharmaceutical formulas, etc. It can solve the problems of insufficient utilization and waste of resources, and achieve shortened effective time, improved absorption rate, and improved stability. The effect of sex and bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Preparation of Maca Microencapsulated Powder
[0029] A. Pulverization: Select high-quality maca medicinal materials, send them into a low-temperature gas pulverizer, and pulverize them through a nitrogen stream at -20°C. The powder passes through a 800-mesh sieve to obtain maca fine powder.
[0030] B. Inclusion: Take the prescribed amount of β-cyclodextrin, add water and grind it evenly, then add the maca fine powder prepared in step A, grind it into a paste and dry it at 5°C to obtain the inclusion product.
[0031] C. Solid dispersion: Add 0.5-5% gas-phase micropowder silica gel by weight of the inclusion product to the inclusion product obtained in step B, mix and grind vigorously for 4-5 hours to disperse evenly and form a solid dispersion.
[0032] D. Microencapsulation: Place the solid dispersion prepared in step C in a fluidized bed, take 0.5-2% sodium carboxymethylcellulose according to the weight ratio of the inclusion product, dissolve it in water, and add...
Embodiment 2
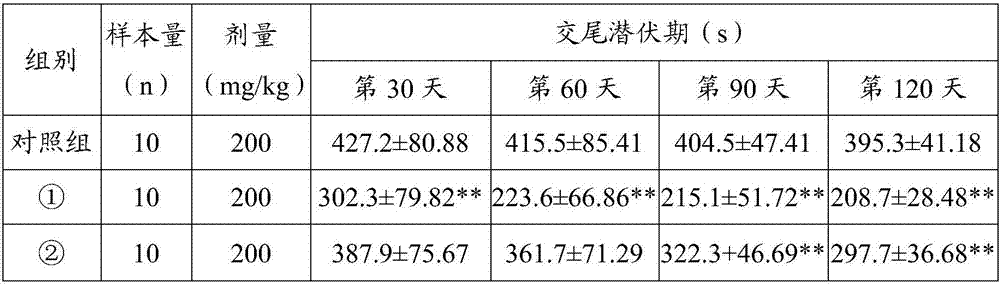
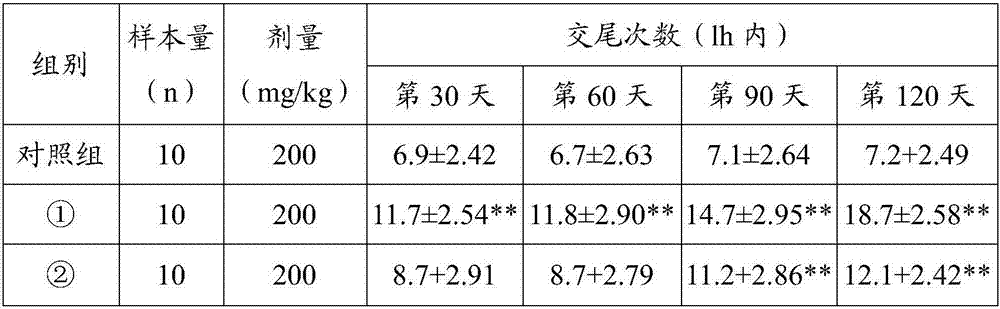
[0034] Effect Experiment of Maca Microencapsulated Powder
[0035] (1) Stability test
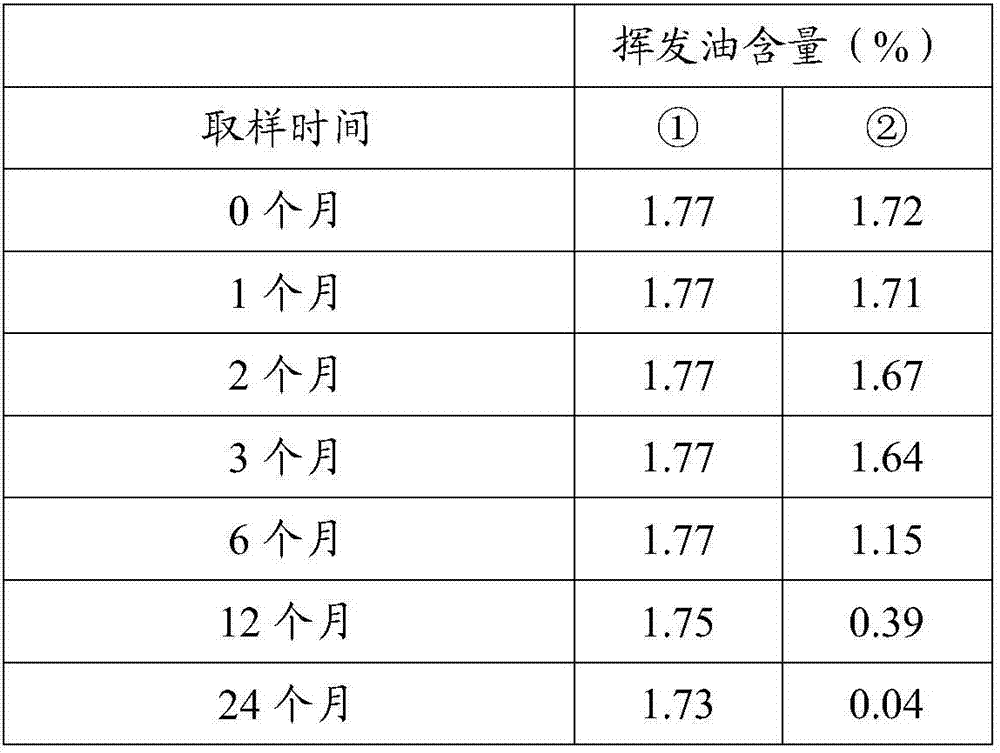
[0036] 1. Sample preparation: Take a sample from the maca microcapsule powder obtained in Example 1, pack it into a 100g / bag, and mark it as No. ①; get ordinary Maca powder, pack it into a 100g / bag, and mark it as No. ②.
[0037] 2. Place the test sample for 6 months at a temperature of 40°C and a relative humidity of 75%, and perform an accelerated stability test (2010 edition of "Chinese Pharmacopoeia", Part II, P Appendix 200), and test samples at 0 months and 1 month respectively. Month, 2 months, 3 months, 6 months sampling, determination of volatile oil content.
[0038]3. Determination method of volatile oil content: Take 10g each of samples ① and ②, add 100mL water, mix well, connect a volatile oil extractor and a reflux condenser, follow the standard method for volatile oil extraction (Part 1 of the 2010 edition of "Chinese Pharmacopoeia", P Appendix 63 ) for extraction, the e...
Embodiment 3
[0072] Preparation of American Ginseng Extract
[0073] Take clean American ginseng stems, leaves and stems, add ethanol aqueous solution with a volume percentage concentration of 70-80% according to the mass-volume ratio (kg / L) of 1:10, and extract at a temperature of 75°C-80°C for 2.5-3 Hours, continuous leaching 3 times. Concentrate the extract obtained by leaching to 1 / 10 of the original volume, put it on a macroporous adsorption resin, first elute it with an ethanol aqueous solution with a volume percentage concentration of 70-80%, and then elute it with water, and collect the eluate. The two eluents obtained by eluting with alcohol and eluting with water are combined, concentrated and dried to obtain American ginseng extract.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com