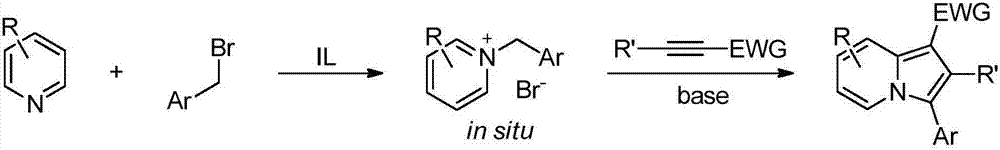
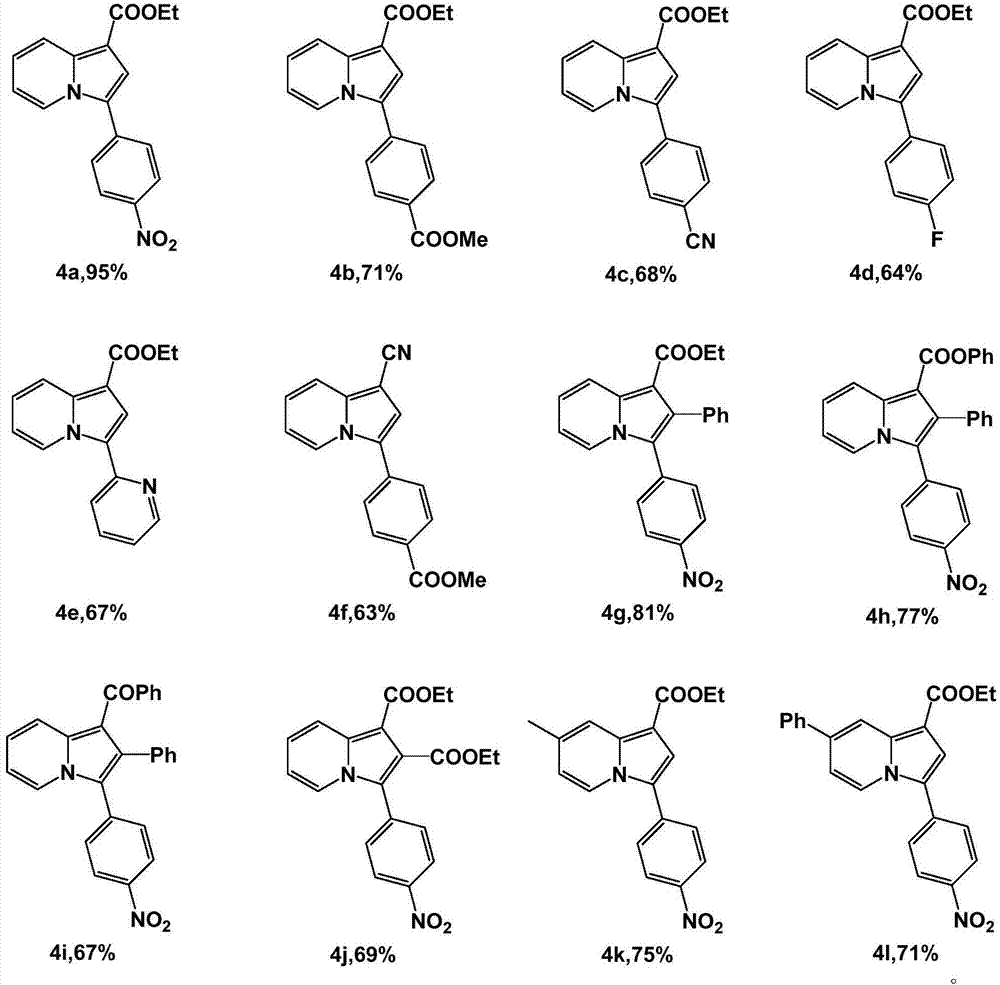
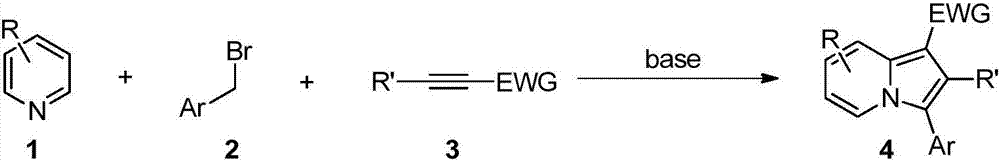Method for synthesizing 3-aryl substituted indolizine
A technology of indolezine and aryl, which is applied in the field of synthesis of 3-aryl substituted indolezine, can solve the problems of increased synthesis cost and post-processing difficulty, high reaction temperature, expensive raw materials, etc., and achieves safe and cheap reaction reagents , The effect of simple reaction operation and simple reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Embodiment 1: Preparation of 3-aryl substituted indolezine 4
[0025]
[0026] Add 0.75mmol of pyridine, 0.75mmol of arylmethyl bromide and 1.0mL of [Omim]Br into a 5mL one-necked flask in sequence, and react at 50°C for 2h. Then 0.5 mmol of electron-deficient alkyne and 0.6 mmol of cesium carbonate were added, and the reaction was continued for 22 h at the same temperature. Cooled to room temperature, extracted three times with ethyl acetate, the obtained ionic liquid phase was directly recovered and used mechanically, the collected organic phase was rotary evaporated to remove the solvent, and finally the target product 3-aryl substituted indolezine 4a-4l was obtained through column chromatography on silica gel. The rates are shown below.
[0027]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com