A kind of pharmaceutical composition and its preparation method and application
A technology for medicines and uses, applied in the field of pharmaceutical compositions, can solve the problems of affecting bioavailability, increasing the solubility and permeability of puerarin, and increasing the absorption of puerarin, so as to increase oral bioavailability and increase intestinal permeability. , the effect of increasing the solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
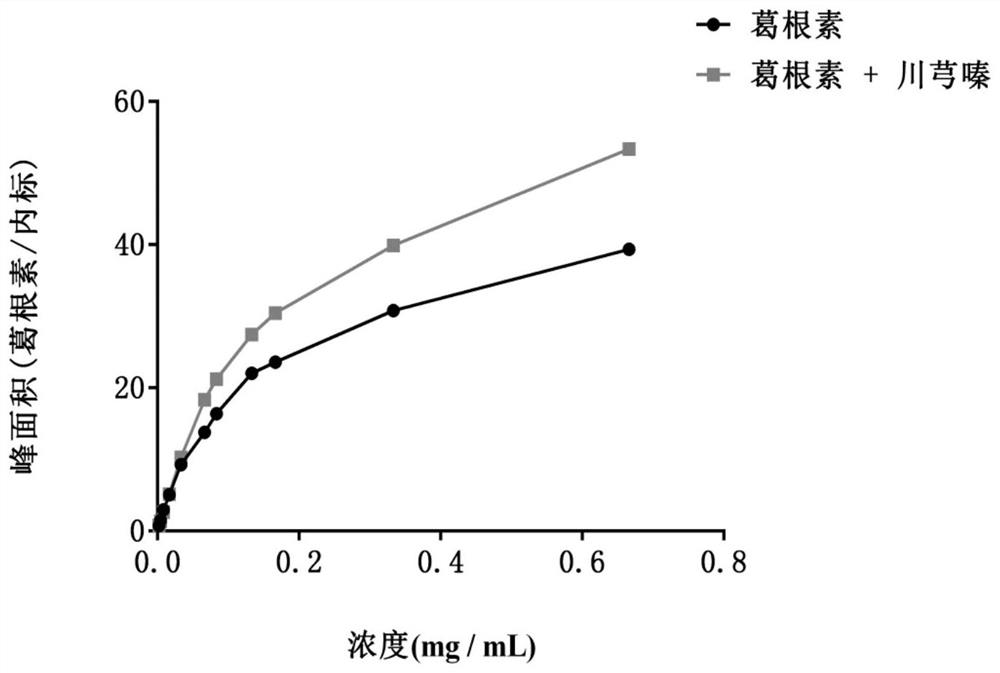
[0025] Embodiment 1 increases the solubility of puerarin with Ligustrazine
[0026] Puerarin solutions of different concentrations (1600, 800, 400, 320, 200, 160, 80, 40, 20, 10, 5 μM) were prepared in HBSS (Hank’s Balanced Salt Solution) medium, and 100 μl of internal standard testosterone solution (100 μM) was added , vortex, and centrifuge. At the same time, prepare the above-mentioned mixed solution of Ligustrazine and Puerarin with concentration gradient in HBSS, the concentration ratio of Ligustrazine and Puerarin is 1:1, add 100 μl internal standard testosterone solution (100 μM), vortex, and centrifuge. The samples were analyzed by ultra-high performance liquid chromatography. The solubility curve was drawn with the theoretical concentration of puerarin as the abscissa and the peak area ratio of puerarin and testosterone as the ordinate. see results figure 1 , the results showed that when the concentration of puerarin in HBSS was low, puerarin dissolved linearly wit...
Embodiment 2
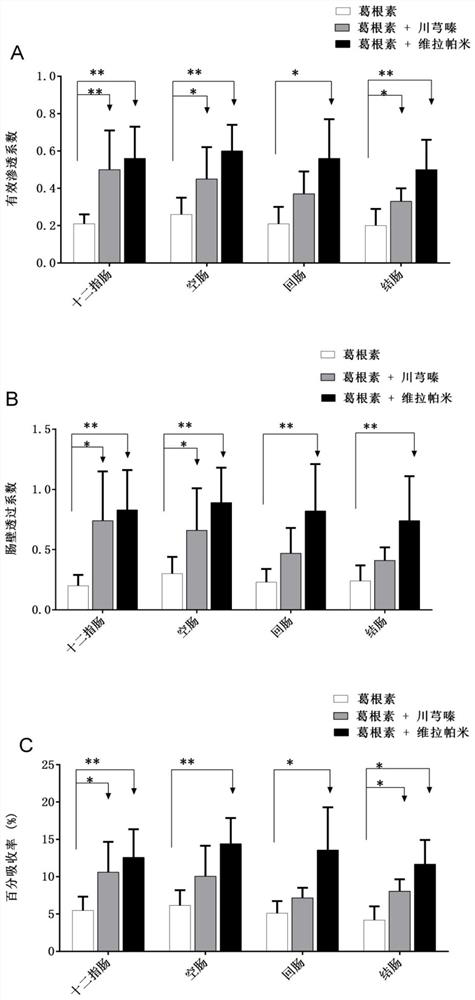
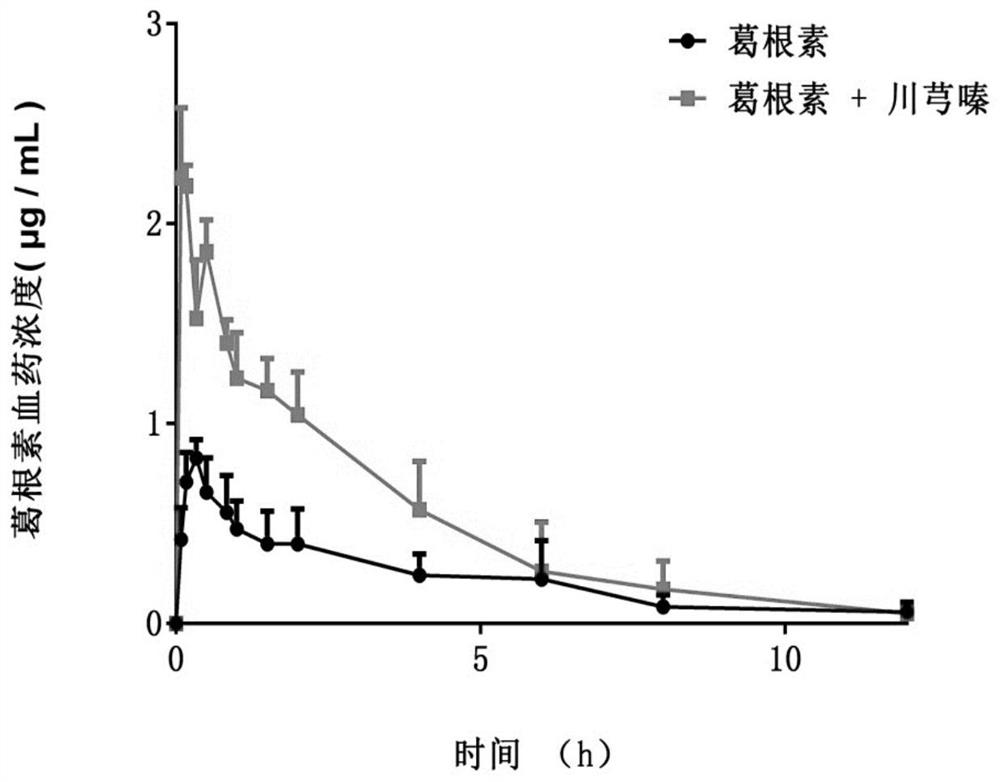
[0027] Example 2 Ligustrazine and Puerarin Medicinal Combination Improve Puerarin Penetration
[0028] The apparent permeability coefficient (Peff) of different concentrations (20μM, 40μM, 160μM) of puerarin in four intestinal segments of rat duodenum, jejunum, ileum and colon was investigated by rat in vivo intestinal perfusion model. The formula for calculating the apparent permeability coefficient is as follows: where Co and Cm are the inlet and outlet concentrations, respectively; Gz=πDL / 2Q, where Q is the perfusion velocity, L is the length of the intestinal segment, and D is the diffusion coefficient.
[0029]The results of the study showed that under the investigated concentration conditions (Co=40μM), the effective permeability coefficient of puerarin in the duodenum, jejunum, ileum, and colon were 0.21±0.05, 0.26±0.09, and 0.21±0.07, respectively. , 0.20±0.09. When ligustrazine and puerarin were used together, the effective permeability coefficients of puerarin in ...
Embodiment 3
[0031] Example 3 The medicinal combination of ligustrazine and puerarin reduces the effect of intestinal transport proteins on the efflux of puerarin. The effect of different concentrations of puerarin on the duodenum, jejunum, ileum, and colon of rats was investigated using a rat in vivo intestinal perfusion model. Percent absorption of four intestinal segments. The results of the study showed that under the investigated concentration conditions, the percentage absorption rate of puerarin in the four intestinal segments of duodenum, jejunum, ileum and colon were 5.47±1.87, 6.15±2.03, 5.11±1.61, 4.20±1.83 . In addition, the absorption of puerarin when puerarin was combined with verapamil (P-gp specific inhibitor) and when puerarin was combined with ligustrazine was investigated. The results showed that when verapamil was combined with puerarin, At the same concentration, the percent absorption rate of puerarin in the duodenum, jejunum, ileum and colon were 12.57±3.77, 14.41±3...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com