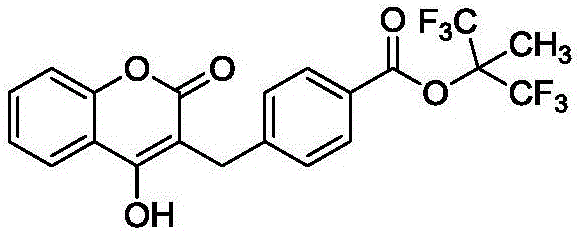
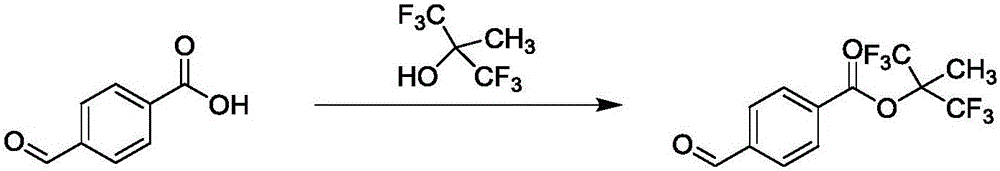
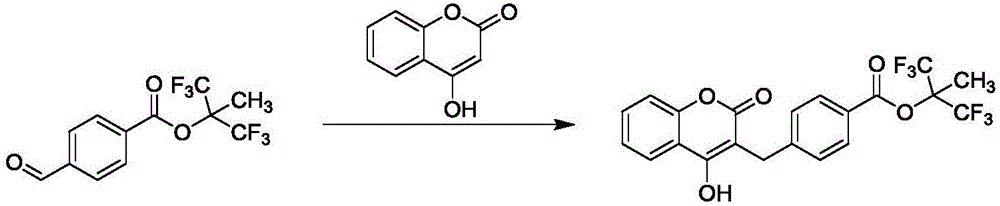
Preparation method of tecarfarin
A technology of ticarfarin and compounds, which is applied in the field of drug synthesis, can solve the problems of many hydrolyzed impurities, difficult to remove, and long reaction time, so as to improve product quality and yield, reduce the production of hydrolyzed impurities, and reduce reaction time Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Embodiment 1: the preparation of compound 1
[0030]
[0031] React 100g (0.66mol) p-aldehyde benzoic acid, 140g (0.86mol) 4-hydroxycoumarin, 500ml formic acid, 200ml triethylamine, 200ml N,N-dimethylacetamide mixed solvent at 120℃ for 1h . After the reaction, cool down to 50°C, add 200ml of 50% ethanol, cool down to 10-20°C for crystallization, filter the feed solution, wash the filter cake with 50% ethanol, and dry to obtain 151g of white compound 1 with a yield of 77%, HPLC Detect product purity 98.8%.
Embodiment 2
[0032] Embodiment 2: the preparation of compound 1
[0033]
[0034] React 100g (0.66mol) of p-aldehyde benzoic acid, 107g (0.86mol) of 4-hydroxycoumarin, 500ml of formic acid, 200ml of triethylamine, and 200ml of N,N-dimethylformamide at 100°C for 1 hour . After the reaction, cool down to 50°C, add 400ml of methanol, cool down to 10-20°C to crystallize, filter the feed liquid, wash the filter cake with methanol, and dry to obtain 140g of white compound 1 with a yield of 72%. The purity of the product detected by HPLC is 98.6%. .
Embodiment 3
[0035] Embodiment 3: the preparation of compound 1
[0036]
[0037] React 100g (0.66mol) p-aldehyde benzoic acid, 160g (0.99mol) 4-hydroxycoumarin, 500ml formic acid, 200ml triethylamine, 200ml N,N-dimethylacetamide mixed solvent at 120°C for 1h . After the reaction, the temperature was lowered to 50°C, 500ml of n-butanol was added, the temperature was lowered to 10-20°C for crystallization, the feed liquid was filtered, the filter cake was washed with n-butanol, and dried to obtain 142g of white compound 1 with a yield of 73%, detected by HPLC The product purity is 98.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com