Metformin gastric retention tablet and preparation method thereof
A metformin and gastric retention technology, applied in the field of medicine, can solve problems such as not being able to meet the requirements of gastric retention time, achieve good market application value, and enrich the effect of product types
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
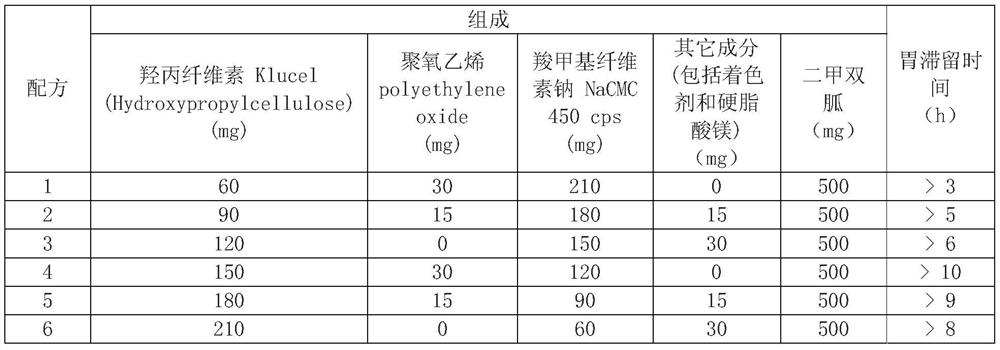
[0008] 1. The floating and swelling synergistic formula contains Klucel (Hydroxypropylcellulose), polyethylene oxide (PEO 8000K), sodium carboxymethylcellulose NaCMC 450cps, other ingredients (including coloring agent and magnesium stearate):
[0009] Formulations containing 500 mg of metformin:
[0010]
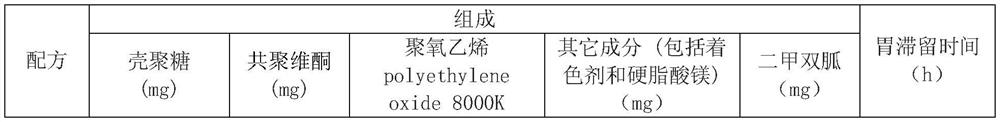
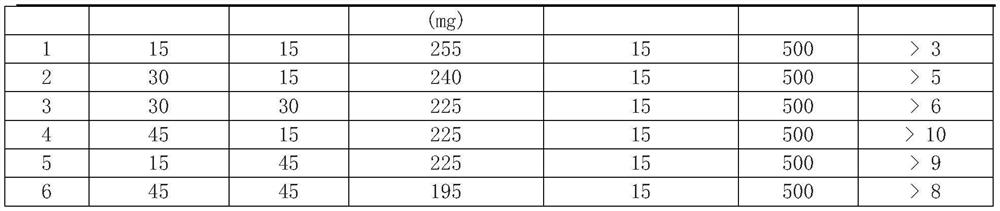
[0011] 2. The bioadhesive swelling synergistic formula contains chitosan, copovidone, polyethylene oxide (PEO 8000K), other ingredients (including coloring agent and magnesium stearate):
[0012] Formulations containing 500 mg of metformin:
[0013]
[0014]
[0015] A tablet preparation method: all excipients (hypromellose HPMC, hypromellose HPC, sodium carboxymethylcellulose NaCMC, polyoxyethylene PEO 8000K, chitosan, copovidone, microcrystalline Cellulose) and magnesium stearate and carbonate pass through No. 60 sieve respectively before tabletting, and then the mixture is compressed with a German Fette tablet machine. The specific tableting method is to use a ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com