Lisinopril compound preparation and preparation method thereof
A technology of compound preparations and excipients, applied in the field of biomedicine, can solve problems such as poor antihypertensive effect, and achieve the effects of being suitable for popularization, controlling blood sugar, and stabilizing blood pressure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0028] The present invention also provides a preparation method of the above lisinopril compound preparation, comprising the following steps:
[0029] a. Dissolve lisinopril in an aqueous povidone solution with a concentration of 5-10%, add starch, gelatin and an appropriate amount of water, stir evenly to make a soft material A, and place it at room temperature for 20-24 hours;
[0030] b. Stir and mix gliquidone, dextrin, microcrystalline cellulose and micropowder silica gel evenly, then add polyethylene glycol, stir evenly to make soft material B for later use;
[0031] c. Take the soft material A prepared in step a and the soft material B prepared in step b at the same dose, press them evenly to form tablets, soak them in a lactose solution with a concentration of 80-90% for 3-5 hours, and dry them to obtain Lisinopril compound preparation.
[0032] Wherein, in the preparation method of the above lisinopril compound preparation, the water content in the soft material A de...
Embodiment 1
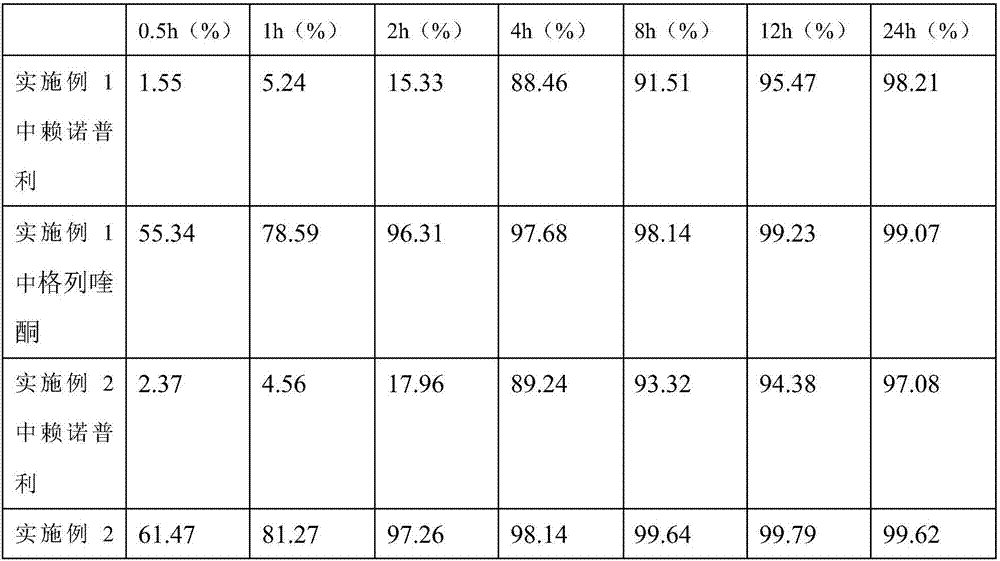
[0040] Embodiment 1 prepares lisinopril compound preparation with the method of the present invention
[0041] The formula of the lisinopril compound preparation consists of: 10 parts of lisinopril, 10 parts of gliquidone, 100 parts of lactose, 30 parts of starch, 70 parts of dextrin, 100 parts of microcrystalline cellulose, poly 20 parts of vitamin C, 30 parts of gelatin, 20 parts of micronized silica gel, and 50 parts of polyethylene glycol.
[0042] Concrete preparation method operation is as follows:
[0043] a. Dissolve lisinopril in a 5% povidone aqueous solution, add starch, gelatin and an appropriate amount of water, stir evenly to make a soft material A, and place it at room temperature for 20 hours;
[0044] b. Stir and mix gliquidone, dextrin, microcrystalline cellulose and micropowder silica gel evenly, then add polyethylene glycol, stir evenly to make soft material B for later use;
[0045]c. Take the soft material A prepared in step a and the soft material B pr...
Embodiment 2
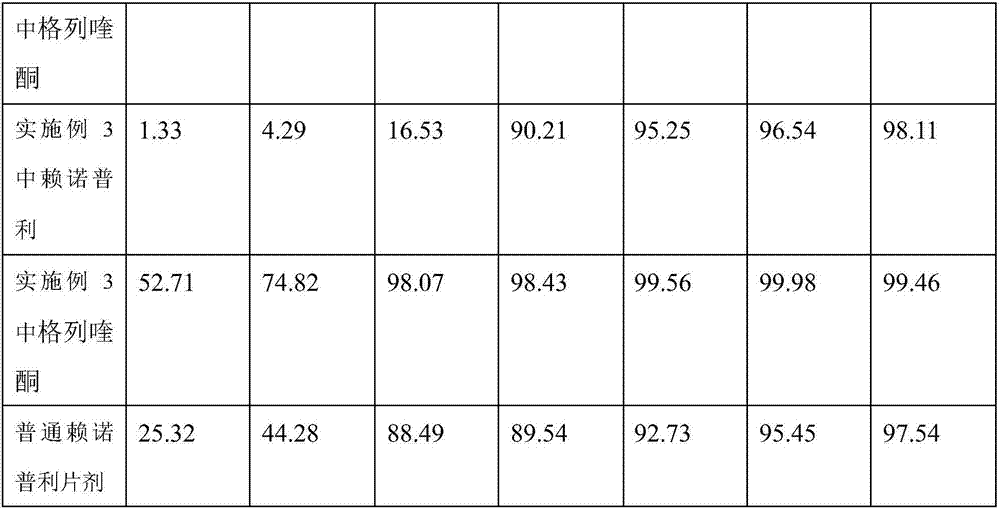
[0046] Embodiment 2 prepares lisinopril compound preparation with the method of the present invention
[0047] The formula of the lisinopril compound preparation consists of: 20 parts of lisinopril, 20 parts of gliquidone, 10 parts of lactose, 10 parts of starch, 20 parts of dextrin, 10 parts of microcrystalline cellulose, poly 10 parts of vitamin C, 20 parts of gelatin, 10 parts of micronized silica gel, 30 parts of polyethylene glycol.
[0048] Concrete preparation method operation is as follows:
[0049] a. Dissolve lisinopril in 10% povidone aqueous solution, add starch, gelatin and appropriate amount of water, stir evenly to make soft material A, and place it at room temperature for 24 hours;
[0050] b. Stir and mix gliquidone, dextrin, microcrystalline cellulose and micropowder silica gel evenly, then add polyethylene glycol, stir evenly to make soft material B for later use;
[0051] c. Take the soft material A prepared in step a and the soft material B prepared in s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com