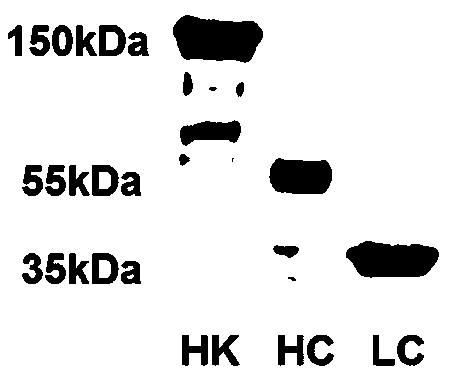A lipopolysaccharide-binding protein polypeptide and its pharmaceutical use
A technology that combines proteins and lipopolysaccharides, applied in the field of biomedicine, can solve the problems of unclear LPS hijacking host proteins and high LPS concentration, and achieve the effects of inhibiting the production of inflammatory factors, good solubility, and reducing mortality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
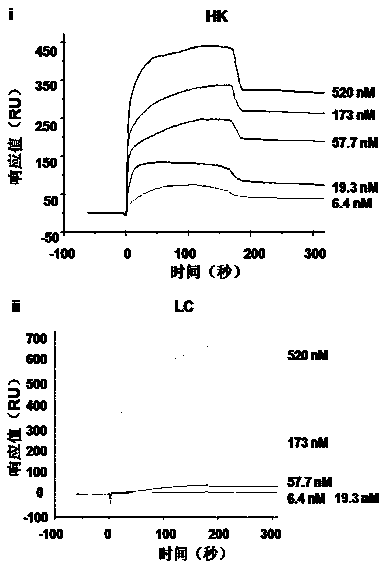
[0023] Example 1: Screening the functional polypeptide DHG15 from plasma HK protein and detecting its binding performance.
[0024] The HK protein in plasma has 6 structural domains, among which the first to third domains (D1~D3) form the heavy chain of HK (Heavy chain, HC), and the fourth to sixth domains (D4~D6) form HK Light chain (Light chain, LC). The heavy chain and light chain proteins of HK were prepared using the Bac-to-Bac insect cell expression system, and the purified HC (55KD) and LC (35KD) proteins were identified by Western blotting. The results are as follows: figure 1 shown.
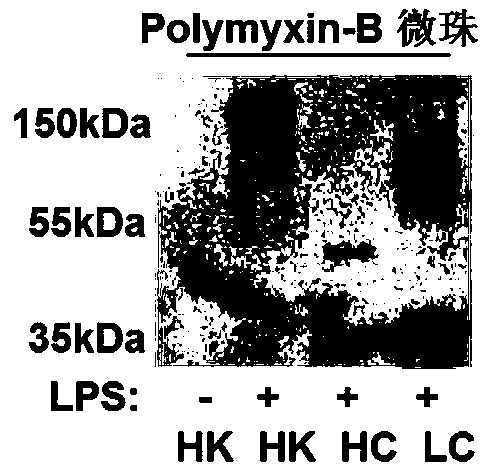
[0025] In order to study the binding site of HK protein and LPS, 200 ng of HK, HC, LC proteins were incubated with LPS or PBS (4°C, 30 min). Pipette 30 μl of mixed Polymyxin-B (Sigma) into a 1.5 ml centrifuge tube, centrifuge to remove the supernatant, wash twice with 1 ml PBS (7000 rpm, 2 min). Incubate various protein samples with the treated Polymyxin-B (4°C, 5min), centrifuge to r...
Embodiment 2
[0033] Example 2: LPS binds to HK through sugar chains.
[0034] LPS is mainly composed of sugar chains and lipid A. In order to detect which part of LPS binds to HK through its structure, 2 μg of lipid A, defatted LPS, LPS or BSA (inner Contains 0.1M Na 2 CO 3 , pH=9.6), coated at 4°C overnight. After washing the well plate with PBS, 1% gelatin was added and blocked at 37°C for 1 h. After washing the plate with PBS, FITC-labeled HK protein (100 nM) was added to each well and incubated at 37°C for 1 h. After aspirating the liquid, use a microplate reader to detect the fluorescence intensity in each well (excitation light: 494 nm, emission light: 518 nm).
[0035] Such as Figure 7 As shown, the binding ability of defatted LPS to HK is the strongest, which is significantly different from that of lipid A and HK, suggesting that the sugar chain on LPS is the main site for the binding of LPS to HK.
Embodiment 3
[0036] Example 3: Investigation of the activity of the DHG15 polypeptide.
[0037] In order to study whether the DHG15 polypeptide can improve the death of mice caused by LPS, the mice were randomly divided into two groups, administered by intraperitoneal injection, and one group was injected with LPS co-incubated with irrelevant polypeptide (200 g / mouse) group), the other group was injected with LPS co-incubated with DHG15 polypeptide (200 g / mouse) (treatment group), and the survival rate of the two groups of mice was observed. It was found that after treatment with DHG15 polypeptide, the mortality rate of mice caused by LPS could be significantly improved.
[0038] In order to investigate whether DHG15 affects the levels of major inflammatory factors in plasma, the levels of major inflammatory factors in plasma were compared between control mice and DHG15-treated mice. Such as Figure 9 As shown, compared with the mice in the control group, the levels of TNF-a, IL-1b and I...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com