Tartaric acid pimavanserin impurities and preparation method thereof
A technology of pimavanserin and tartaric acid, which is applied in the field of drug synthesis and can solve problems such as no relevant reports on the synthesis method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
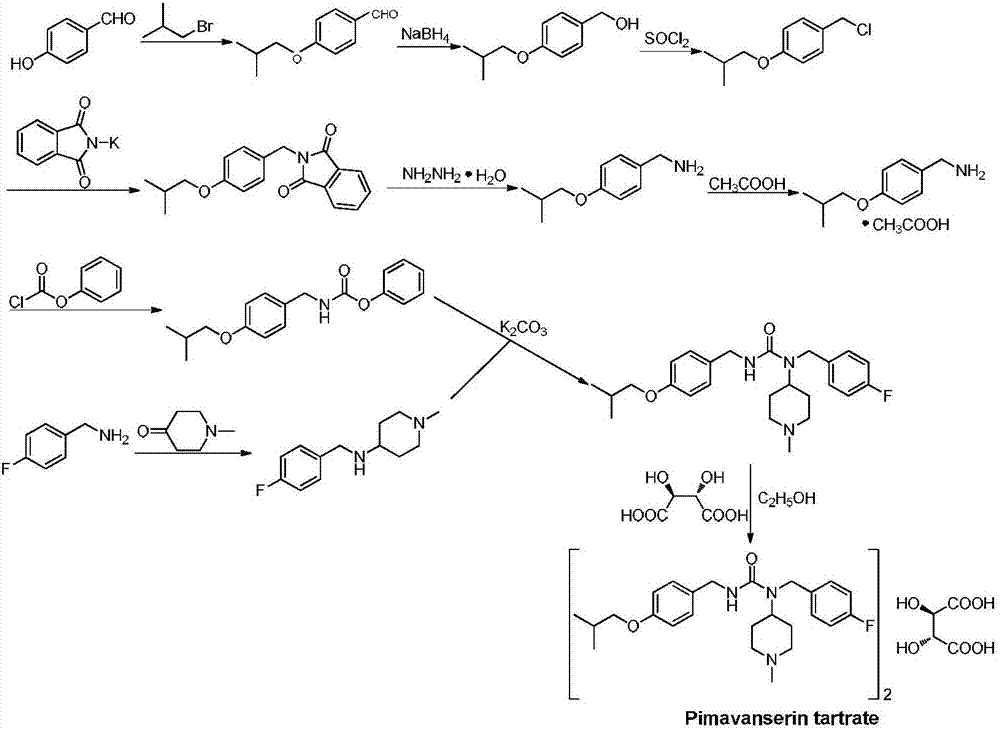
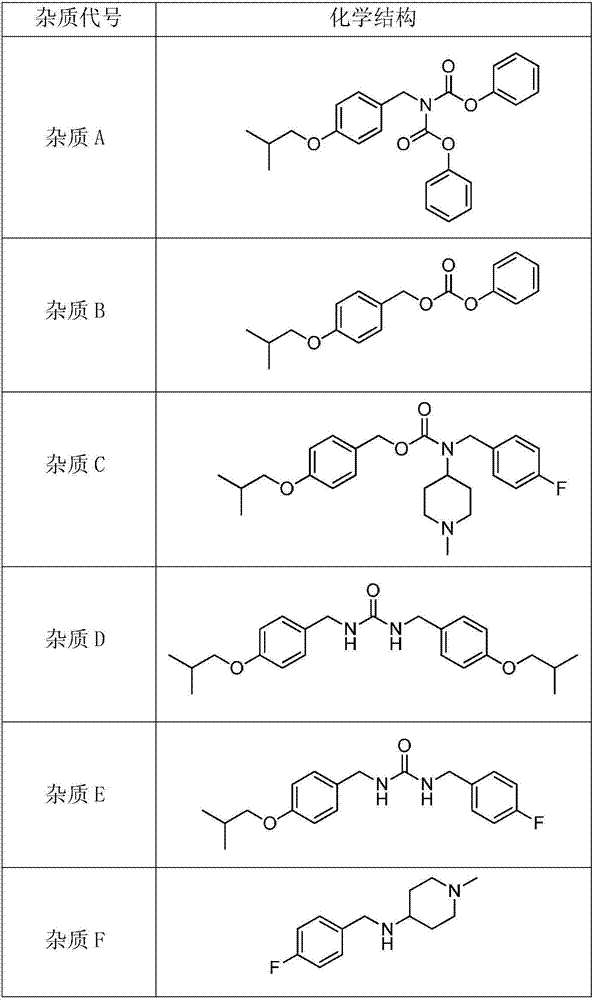
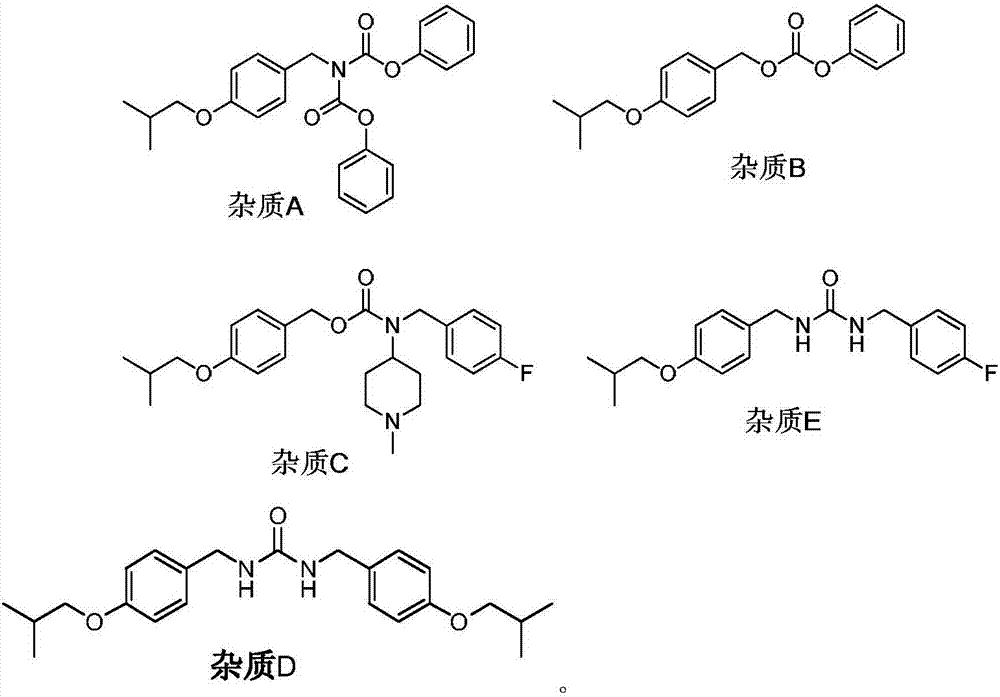
[0052] Embodiment 1: Preparation of N-(4-isobutoxybenzyl) diphenyl iminodicarboxylate (impurity A)
[0053]Add 5.2g (0.029mol) 4-isobutoxybenzylamine, 11.7g (0.116mol) triethylamine, 3.5g (0.029mol) 4-dimethylaminopyridine (DMAP) to 300ml dichloromethane at room temperature , and cooled the solution to -5°C-0°C. 11.3 g (0.072 mol) of phenyl chloroformate was added dropwise at -5°C-0°C, and after 40 minutes, the solution was transferred to room temperature and heated to 40°C for 12 hours. After completion of the reaction, the reaction solution was washed with 400ml × 2 (8%) dilute hydrochloric acid aqueous solution, separated into layers, the organic layer was washed with 300ml water, 300ml × 2 saturated brine, the organic layer was dried over anhydrous sodium sulfate, and the solvent was removed by rotary evaporation under reduced pressure to obtain 11.5 g pale yellow solid crude product, add 60ml petroleum ether and 8ml ethyl acetate, recrystallize at 60°C to obtain 7.8g whi...
Embodiment 2
[0054] Embodiment 2: Preparation of 4-isobutoxybenzyl phenyl carbonate (impurity B)
[0055] Add 50.0g (0.41mol) p-hydroxybenzaldehyde, 84.8g (0.62mol) potassium carbonate, 2.0g (0.012mol) potassium iodide to 200ml N,N-dimethylformamide at room temperature, stir at room temperature for 10min, add 111.4g (0.82mol) of bromoisobutane was heated to 83°C for 24h. After the reaction is complete, filter with suction, pour the filtrate into 300ml of 4% sodium hydroxide aqueous solution and stir for 5min, extract twice with 350×2 ethyl acetate, combine the organic layers, wash the organic layer with 500ml×2 saturated saline, and dry over anhydrous sodium sulfate , evaporate the solvent under reduced pressure to obtain 64.6g light yellow oil crude product 4-isobutoxybenzaldehyde, yield: 88.5%,
[0056] Add 27.6g (0.73mol) of sodium borohydride to 300ml of tetrahydrofuran at room temperature, cool the mixture to 0°C, add 64.6g (0.36mol) of crude 4-isobutoxybenzaldehyde to 100ml of tetra...
Embodiment 3
[0058] Example 3: Preparation of 4-fluorobenzyl (1-methylpiperidin-4-yl)carbonate-4-isobutoxybenzyl ester (impurity C) by method 1
[0059] Method 1: 15.0g (0.035mol) 4-isobutoxybenzyl phenyl carbonate (impurity B) crude product, 7.8g (0.035mol) N-(4-fluorobenzyl)-1-methyl - Add 4-piperidine and 8.0g (0.058mol) potassium carbonate to 80ml N,N-dimethylformamide, heat up to 50°C and react for 5h. After the reaction was completed, filter with suction, add 100ml of water, extract with 150ml×2 ethyl acetate, wash the organic layer with 300ml×3 saturated brine, dry over anhydrous sodium sulfate, and distill off the solvent under reduced pressure to obtain 10.5g of crude yellowish-brown oily product, yield 70.0 %. The crude product was purified by silica gel column chromatography (petroleum ether: ethyl acetate = 10:1) to obtain a colorless transparent liquid. ESI-MS:429.4[M+H] + , 451.3[M+Na] + , 1 H NMR (600MHz, DMSO-d 6 ): δ7.25 (dd, J=8.4Hz, 5.4Hz, 2H, ArH), 7.11 (d, J=8.4H...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com