Preparation method of antitumor drug AZD9291
An anti-tumor drug and intermediate technology, which is applied in the field of preparation of anti-tumor drug AZD9291, can solve the problems of long synthesis route, low total yield, unsuitable for industrial production and the like, and achieves high total yield, convenient operation and easy industrialization. production effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] Embodiment 1: the preparation of compound intermediate 1 to intermediate 3
[0053] 1) Preparation of intermediate 1:
[0054] Add 40g (0.164mol) of SM1, 30.6g (0.164mol) of SM2 and 62.3g (0.328mol) of p-toluenesulfonic acid monohydrate into 200ml of isobutanol, heat up to reflux, react for 6h, a yellow solid precipitates out, and drops to After filtering at room temperature, washing with isopropanol, and vacuum drying at 50°C, 54.8 g of a yellow solid was obtained, with a yield of 85.0%.
[0055] 2) Preparation of intermediate 2:
[0056] Method 1: 50g (0.127mol) intermediate 1, 15.0g (0.147mol) N,N,N'-trimethylethylenediamine, 1g cuprous bromide and 19.65g (0.152mol) N,N-di Add isopropylethylamine to 350ml of N,N-dimethylformamide, rise to 100°C and react for 3 hours, cool down to room temperature and filter to remove cuprous bromide, add 80ml of water, a large amount of solids precipitate, filter, wash with water, 45 °C and dried under vacuum to obtain 57.2 g of o...
Embodiment 2
[0060] Embodiment 2: Synthesis of AZD9291
[0061] Add 3.37g (28mmol) of pivaloyl chloride and 4.13g (30mmol) of potassium carbonate to 60ml of 1,4-dioxane, add 2.02g (28mmol) of acrylic acid to the above solution at 0°C, and stir for 1h to prepare Mixed anhydride solution, 6.17g (13.8mmol) of intermediate 3 prepared in Example 1 was added to the mixed anhydride solution at 0°C, and the disappearance of the raw material was monitored by TLC. Add water and stir for 0.5 h, extract with 100 ml of ethyl acetate, dry the organic phase over anhydrous sodium sulfate, filter, and concentrate the filtrate in vacuo at 45°C to obtain crude AZD9291. Recrystallized with 50ml of acetonitrile to obtain 6.29g of brown solid; yield 91.3%, purity 99.8%.
[0062] The preparation of intermediate 2 is calculated by method 1, and the total yield of product is 71.7%. The preparation of intermediate 2 is calculated by method 2, and the total yield of product is 63.2%.
Embodiment 3
[0063] Embodiment 3: the synthesis of AZD9291
[0064] Add 3.2g (28mmol) of methanesulfonyl chloride and 2.22g (30mmol) of lithium carbonate to 100ml of acetonitrile, stir at 10°C, add 2.02g (28mmol) of acrylic acid to the above solution, and stir for 1.5h. 6.17g (13.8mmol) of intermediate 3 was added to the mixed anhydride solution at 0°C, and the disappearance of the raw material was monitored by TLC. Add water and stir for 1 h, extract with 100 ml of ethyl acetate, dry the organic phase over anhydrous sodium sulfate, filter, and concentrate the filtrate in vacuo at 45°C to obtain crude AZD9291. Recrystallize with 50ml of acetonitrile to obtain 6.35g of brown solid, yield 92.2%, purity 99.8%, HPLC spectrum as Figure 5 shown. The preparation of intermediate 2 is calculated by method 1, and the total yield of product is 72.4%. The preparation of intermediate 2 is calculated by method 2, and the total yield of product is 63.8%.
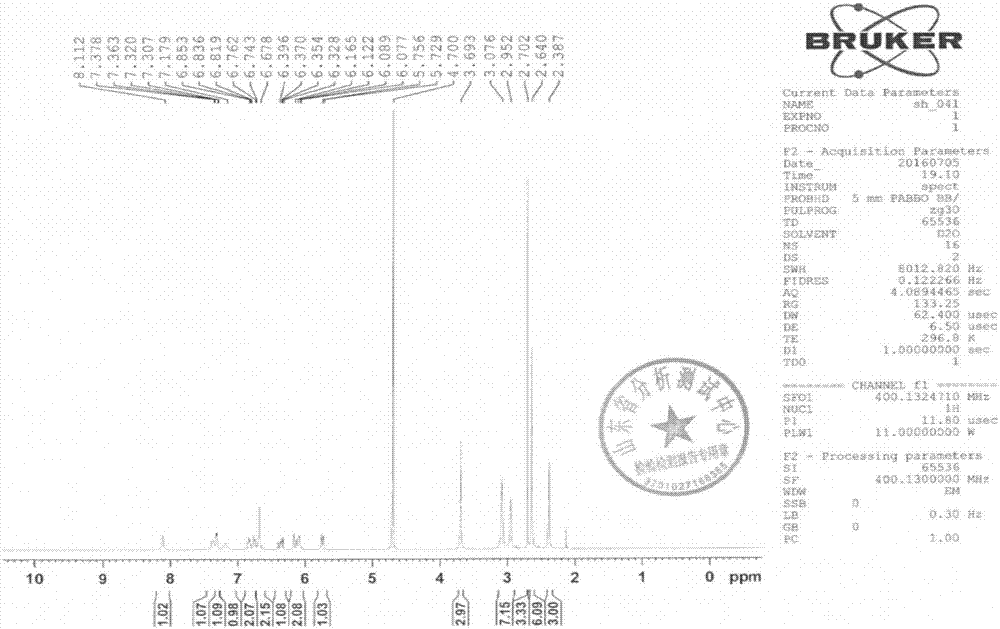
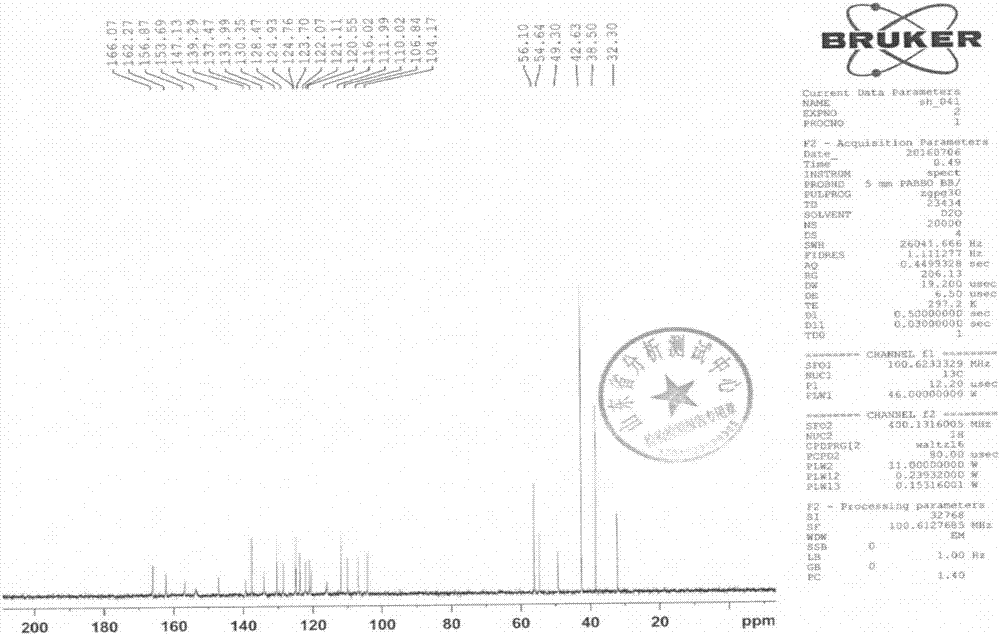
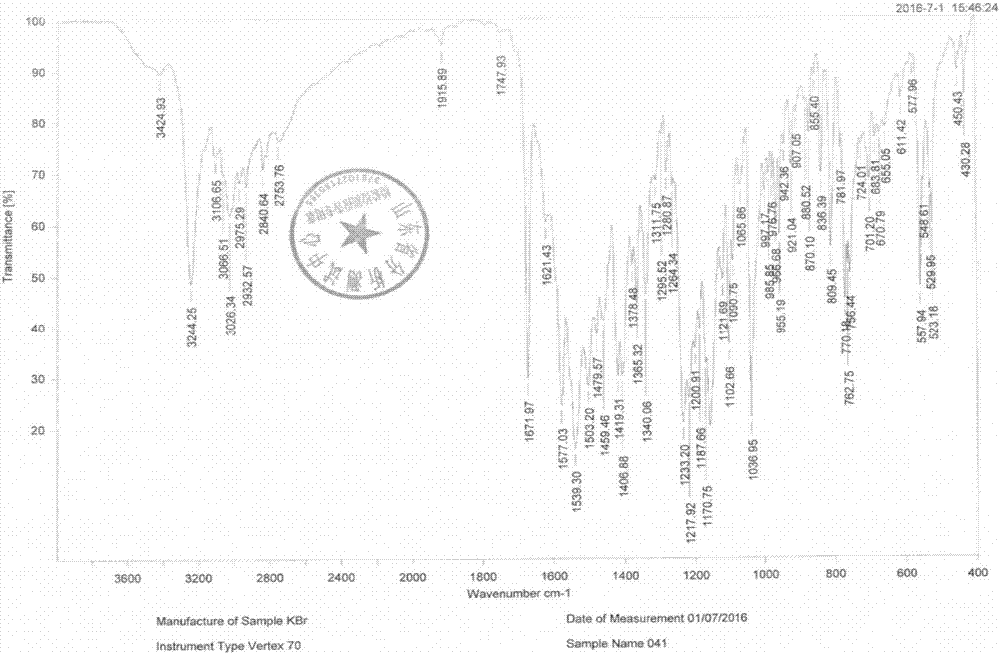
[0065] That 1 H NMR, 13 C-NMR, IR and ESI...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com