Preparation method of phase-change type LiFeSO4F cell material stable at high temperature, electrode plate, and usage of lithium ion cell
A lithium-ion battery, high temperature stable technology, applied in battery electrodes, secondary batteries, secondary battery repair/maintenance, etc. Erosion, effect of high capacity retention
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
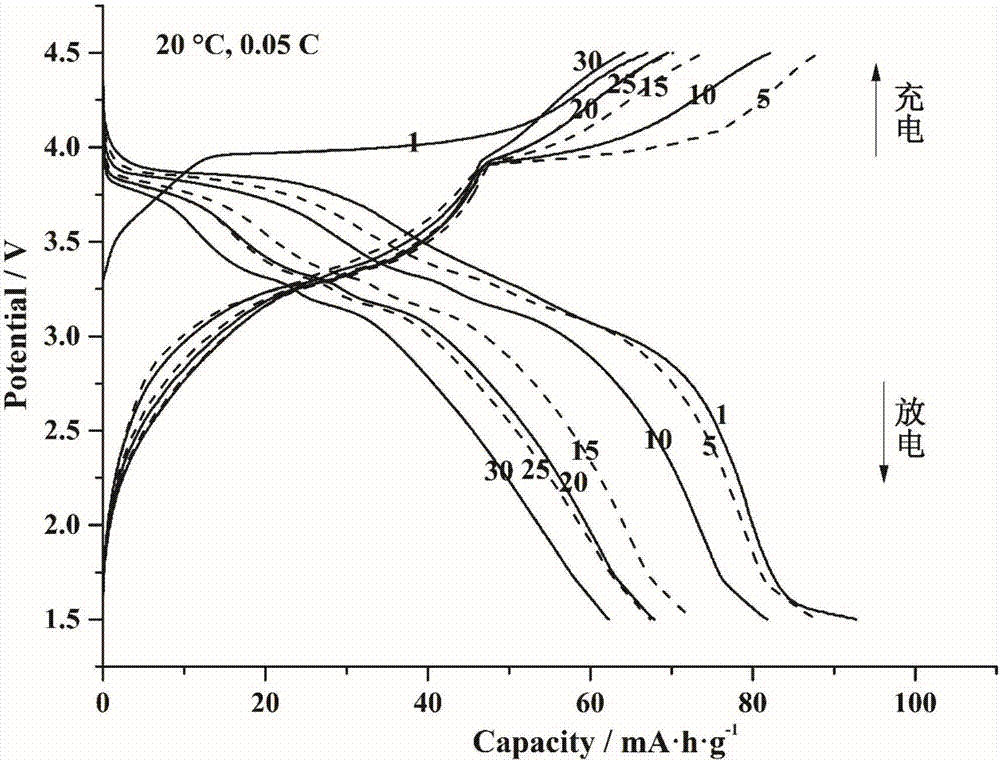
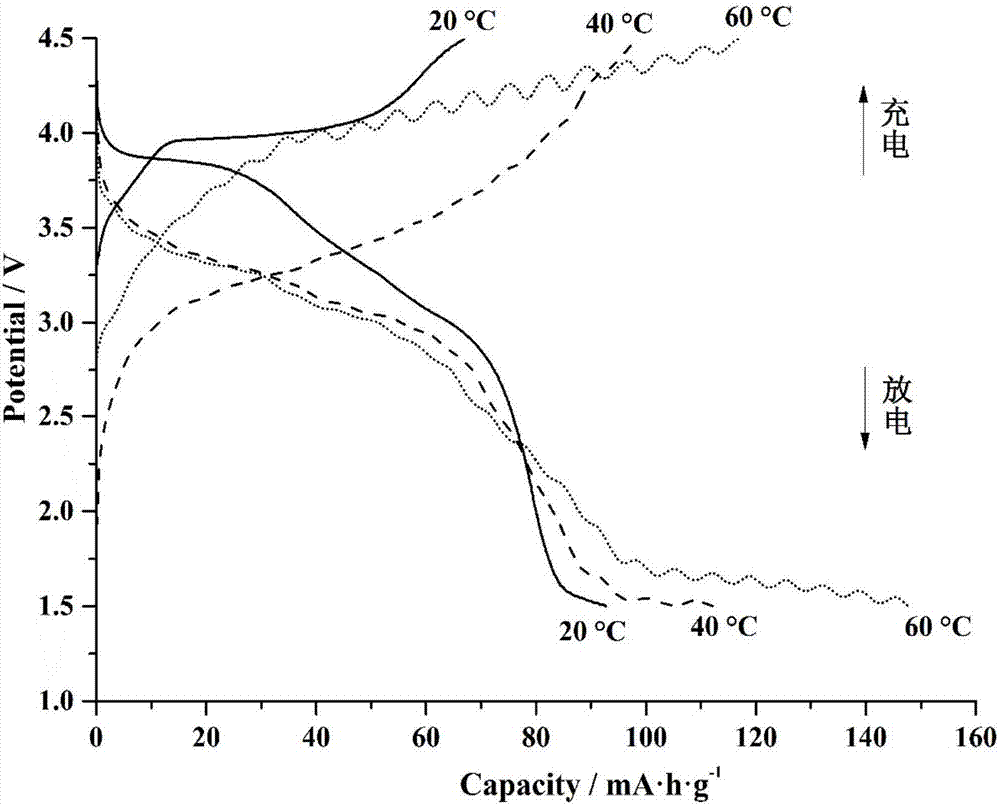
[0043] A high-temperature stable phase-change lithium iron fluorosulfate battery material and a preparation method thereof, comprising the following steps:
[0044] S1.1 by FeSO 4 Measuring ratio to weigh FeSO 4 ·7H 2 O powder, ground, placed in a tube furnace and calcined in argon at 390 °C for 2 hours to obtain FeSO 4 powder.
[0045] S1.2 Weigh the FeSO prepared in step S1.1 according to the molar ratio of 1:1.05 4 and LiF, using ethanol as a medium in a planetary ball mill for 2 hours (the mass ratio of the mixture and ethanol is 3:1), to obtain a slurry, vacuum drying, to obtain FeSO 4 / LiF mixed powder. The mixed powder was calcined in an argon furnace at 450°C for 0.75 hours and ground to obtain LiFeSO 4 F pure phase powder.
[0046] S1.3 the LiFeSO obtained in step S1.2 4 F pure phase powder and nano-conductive carbon (nano-SP) were mixed and ball-milled at a mass ratio of 3.5:1 to obtain uniform LiFeSO 4 F / C carbon coated powder.
[0047] S1.4 LiFeSO obtaine...
Embodiment 2
[0056] FeSO in embodiment two 4 The powder, carbon source, electrolyte, negative electrode sheet and separator are the same as those in Example 1, and the calcination time for synthesizing lithium iron fluorosulfate is different.
[0057] S2.1 will obtain FeSO by embodiment one 4 / LiF mixed powder was calcined in an argon furnace at 450°C for 2.25 hours and ground to obtain LiFeSO 4 F pure phase powder.
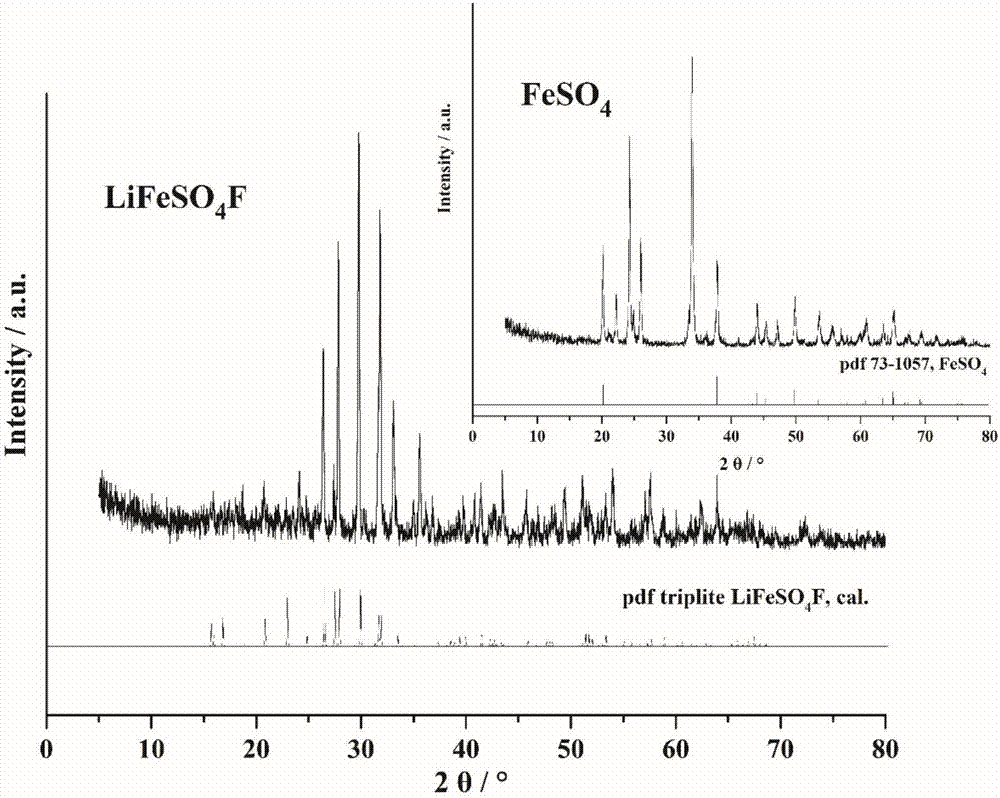
[0058] Figure 7 For adopting the LiFeSO prepared in embodiment two 4 XRD pattern of F powder. The results demonstrate the synthesis of pure-phase LiFeSO 4 F.
Embodiment 3
[0060] FeSO in embodiment three 4 The powder, carbon source, electrolyte, negative electrode sheet and separator are the same as those in Example 1, and the calcination temperature for synthesizing lithium iron fluorosulfate is different.
[0061] S3.1 will obtain FeSO by embodiment one 4 / LiF mixed powder was calcined in an argon furnace at 500°C for 0.75 hours and ground to obtain LiFeSO 4 F pure phase powder.
[0062] Figure 8 For adopting the LiFeSO prepared in Example 3 4 XRD pattern of F powder. The results demonstrate the synthesis of pure-phase LiFeSO 4 F.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com