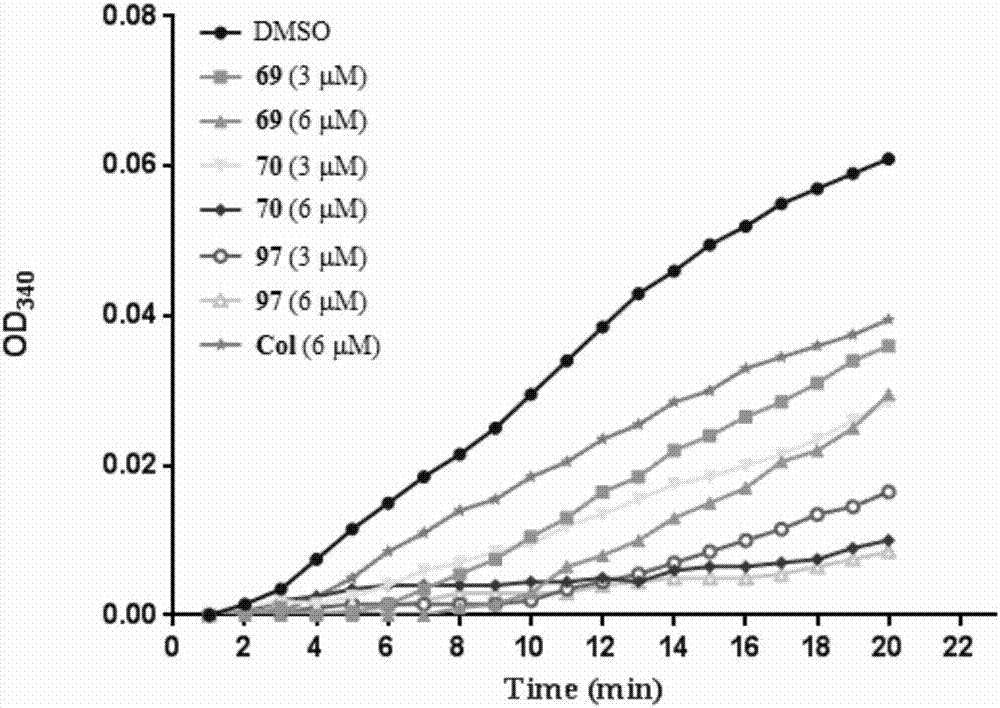
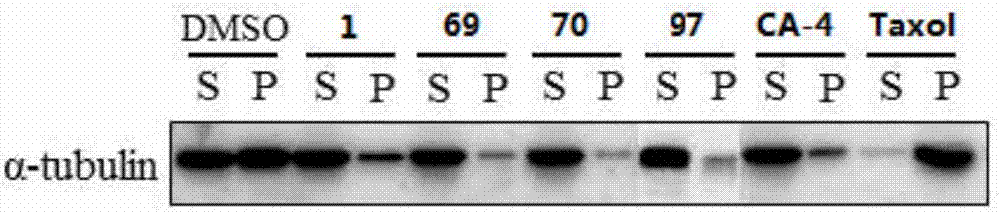
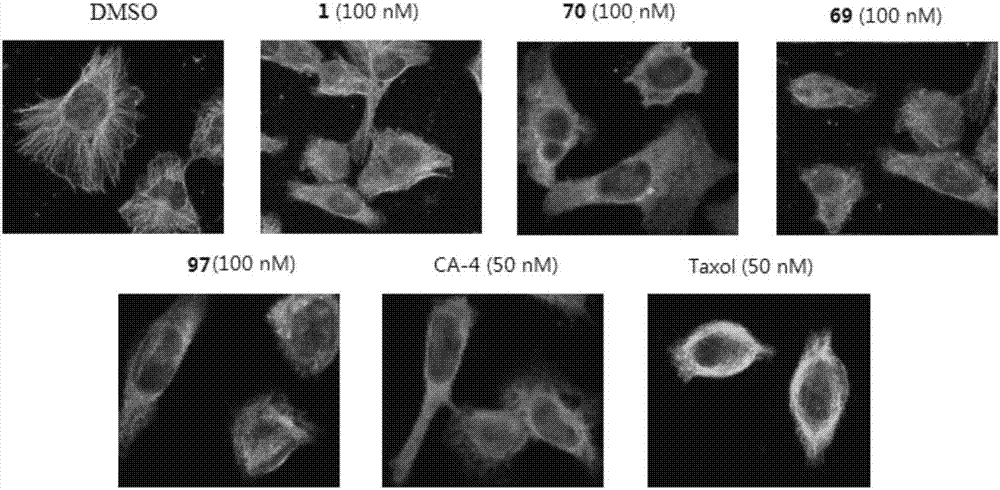
Diaryl-beta-lactam compounds, and preparation method and application thereof in drug preparation
A technology of lactams and compounds, applied in the field of chemical pharmacy, can solve the problems of few compounds and toxicity, and achieve the effect of good tumor growth
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0065] Example 1 Compound (S)-1-(3,4,5-trimethoxyphenyl)-4-(3-hydroxy-4-methoxyphenyl)-3-methyleneazetidine Synthesis of -2-one (1)
[0066] References (Wang, X.; Meng, F.; Wang, Y.; Han, Z.; Chen, Y.-J.; Liu, L.; Wang, Z.; Ding, K. Angewandte Chemie Int. Ed. 2012,124,9410-9416.) method, the present invention synthesizes target compound 1 according to the following route:
[0067]
[0068] 1.1 Synthesis of 3-tert-butyldimethylsilyloxy-4-methoxybenzaldehyde (1b)
[0069] Add 3-hydroxy-4-methoxybenzaldehyde (1a) (1.58g, 10.4mmol), DMAP (25mg, 0.2mmol), anhydrous DCM (50mL) and anhydrous triethylamine (2mL, 14.5mmol), cooled to an ice bath, TBSCl (1.88g, 12.5mmol) was dissolved in anhydrous DCM (10mL), and added slowly from the dropping funnel. Remove the ice bath after dropping, stir at room temperature for two hours, add saturated aqueous sodium bicarbonate solution to quench the reaction, separate the organic phase, extract the aqueous phase with DCM three times, combine...
Embodiment 2
[0080] Example 2 (S)-1-(3,4,5-trimethoxyphenyl)-4-(3-methoxy-4-methoxyphenyl)-3-methyleneazetidine Synthesis of alkan-2-ones (2)
[0081]
[0082] Add anhydrous DMF (1mL), compound 1 (22mg, 0.059mmol) and potassium carbonate (15mg, 0.108mmol) into a 20mL eggplant-shaped bottle, stir at room temperature for 10min, the solution is yellow at this time, add iodomethane (17mg, 0.12mmol) , after stirring and reacting for 3h, TLC (developing solvent: petroleum ether / ethyl acetate=1:1) showed that raw material 1 (R f =0.3) disappear completely, and new points are generated (R f = 0.5). To stop the reaction, 10 mL of ethyl acetate was added, washed with water (2 x 10 mL), washed with saturated brine (10 mL), and dried over anhydrous sodium sulfate. Mixed with silica gel column chromatography for separation and purification, the corresponding eluent was collected, and the solvent was evaporated to dryness to obtain 23 mg of white solid (2), with a yield of 97%. Mp 138-139°C; [α] ...
Embodiment 3
[0083] Example 3 (S)-1-(3,4,5-trimethoxyphenyl)-4-(3-ethoxy-4-methoxyphenyl)-3-methyleneazetidine Synthesis of alkan-2-ones (3)
[0084]
[0085] Add 1mL of anhydrous DMF, compound 1 (22mg, 0.059mmol) and potassium carbonate (15mg, 0.108mmol) into a 20mL eggplant-shaped bottle, stir at room temperature for 10min, the solution is yellow at this time, add bromoethane (12mg, 0.12mmol) , overnight reaction, TLC (developing solvent: sherwood oil / ethyl acetate=1:1) shows raw material 1 (R f =0.3) disappear completely, and new points are generated (R f =0.5), stop the reaction, add 10 mL of ethyl acetate, wash with water (3 x 10 mL), and dry over anhydrous sodium sulfate. It was directly mixed with silica gel column chromatography for separation and purification, the corresponding eluent was collected, and the solvent was evaporated to dryness to obtain 23 mg of white solid (3) with a yield of 97%. Mp 92-93°C; [α] D 20 =+34.0(c 0.27, CHCl 3 ). 1 HNMR (400MHz, CDCl 3 )δ6.98...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com