Method for detecting ammonia nitrogen of low mercury salt
A detection method, ammonia nitrogen technology, applied in the field of ammonia nitrogen detection of low-mercury salts, can solve problems such as threats to human health, improper recycling, and environmental pollution, and achieve fast and simple detection, high accuracy and precision, and high sensitivity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
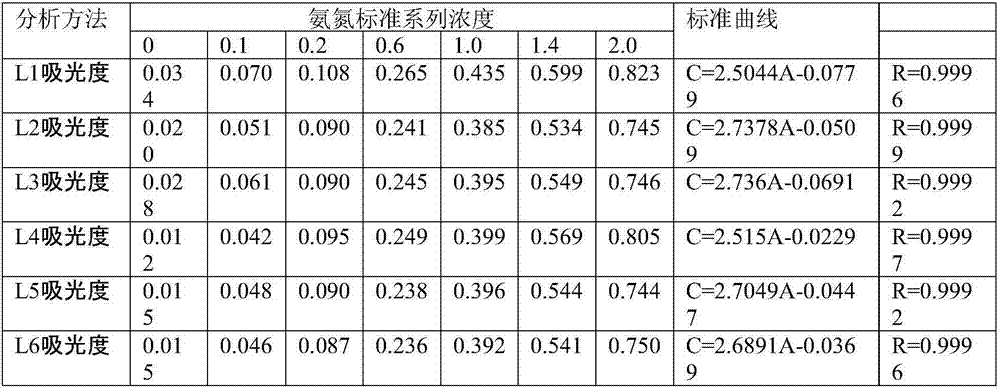
[0025] Potassium sodium tartrate is prepared according to the national standard: Weigh 50g of potassium sodium tartrate and dissolve it in 100ml of water, heat to boil to remove ammonia, let cool to room temperature, add water to make up to 100ml. The mass concentration of the prepared potassium sodium tartrate is ρ=500g / l.
[0026] Nessler's reagent is prepared by the method required by the national standard: mercuric dichloride-potassium iodide-potassium hydroxide (HgC1 2 -KI-KOH) solution: Weigh 15.0g of potassium hydroxide (KOH), dissolve it in 50ml of water, and cool to room temperature; weigh 5.0g of potassium iodide (KI), dissolve it in 10ml of water, and dissolve 2.50g of dichloro Mercury (HgCl 2 ) powder was added to the potassium iodide solution several times, and 0.8gHgCl was added for the first time 2 The powder dissolution time is 1min, completely dissolved, the second time 0.5gHgCl 2 Powder, the dissolution time is 1.5min, add 0.5gHgCl for the third time 2 Th...
Embodiment 2
[0031] Potassium sodium tartrate is prepared according to the national standard: Weigh 50g of potassium sodium tartrate and dissolve it in 100ml of water, heat to boil to remove ammonia, let cool to room temperature, add water to make up to 100ml. The mass concentration of the prepared potassium sodium tartrate is ρ=500g / l.
[0032] Nessler's reagent preparation, using the rapid method Nessler's reagent preparation: Weigh 16g of sodium hydroxide, dissolve in 50ml of water, fully cool to room temperature. Another weighed 7g potassium iodide and 10g mercuric iodide (HgI 2 ) dissolved in water, then slowly pour this solution into sodium hydroxide solution under stirring, dilute it to 100ml with water, store it in a polyethylene bottle, and keep it sealed.
[0033] In seven 50ml colorimetric tubes, add 0ml, 0.5ml, 1.0ml, 3.0ml, 5.0ml, 7.0ml and 10.0ml of ammonia nitrogen standard working solution respectively, the mass concentration of ammonia nitrogen standard working solution i...
Embodiment 3
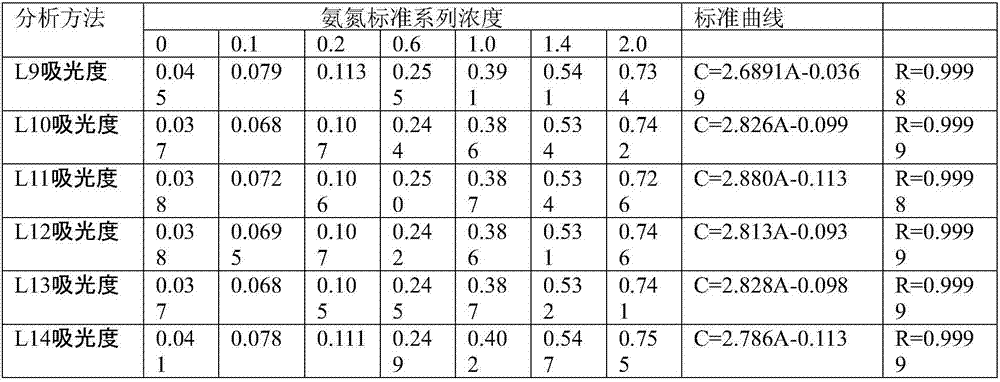
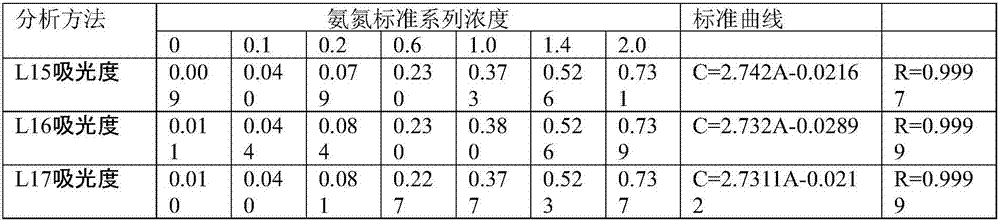
[0039] Step 1, preparation of mercuric dichloride-potassium iodide-potassium hydroxide solution is Nessler's reagent
[0040] Weigh 15.0g of potassium hydroxide, dissolve in 50ml of water, cool to room temperature for later use;
[0041] Weigh 5.0g of potassium iodide, dissolve it in 10ml of water, and add 2.50g of mercuric dichloride powder into the potassium iodide solution several times while stirring. Add 0.8g of mercuric dichloride powder for the first time, and the dissolution time is 1min. Add 0.5g, dissolving time is 1.5min, add 0.5g for the third time, dissolving time is 3min, add 0.3g for the fourth time, dissolving time is 5min, add 0.2g for the fifth time, dissolving time is 5min, add for the sixth time 0.1g, the dissolution time is 8min, add 0.05g for the seventh time, the dissolution time is 15min, add 0.05g for the eighth time, the dissolution time is 30min, the mercuric dichloride powder is not completely dissolved, stop adding the mercuric dichloride powder; ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Absorbance | aaaaa | aaaaa |
| Absorbance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com