Preparation method of oxygen evolution reaction catalyst
An oxygen evolution and catalyst technology, applied in the field of electrochemical catalysis, can solve the problems that the catalyst cannot achieve the ideal catalytic effect, difficult to achieve large-scale preparation, and high reaction overpotential, and achieves the effects of non-toxic control, low cost, and improved catalytic performance.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
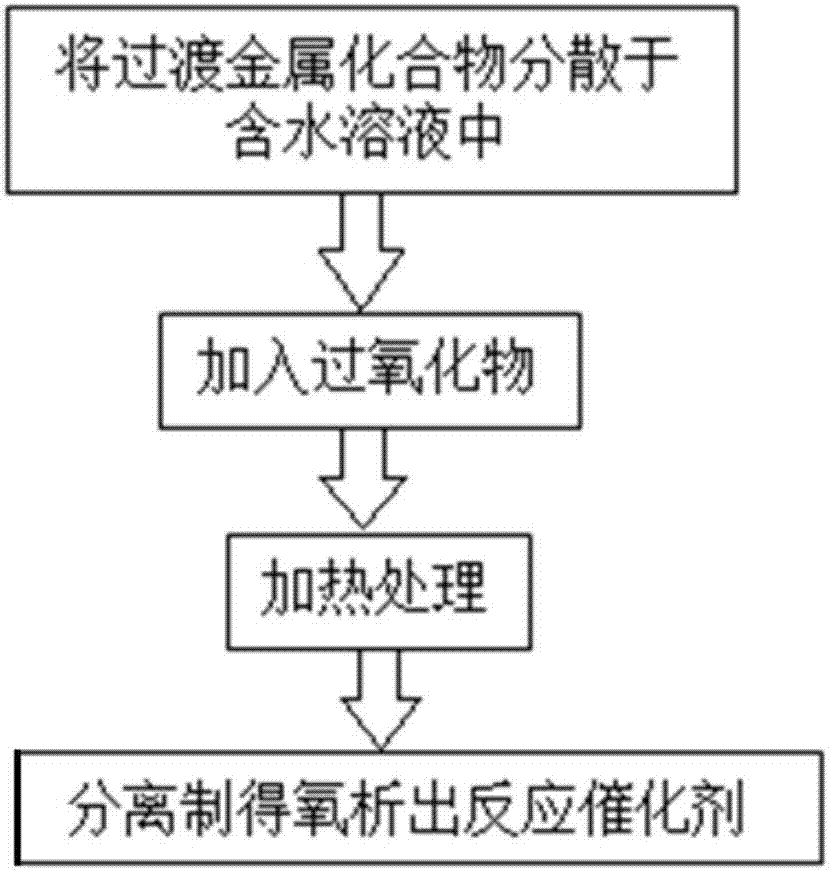
[0029] Such as figure 1 Shown, be the preparation method of oxygen evolution reaction catalyst of the present invention, this method comprises the following steps:
[0030]S1 will have two or more transition metal compounds (preferably oxides, sulfides, phosphides, selenides, carbides or nitrides of transition metals such as Fe, Co, Ni, Mn) with 0.1g / The mass concentration of L-5000g / L is dispersed in aqueous solution (preferably pure water, potassium chloride solution, sodium chloride solution, potassium hydroxide solution, sodium hydroxide solution, potassium sulfate solution or sodium sulfate solution), and ultrasonically mixed Uniform;
[0031] S2 adds peroxide (preferably: hydrogen peroxide, acetylacetone peroxide, 2,2-bis-(tert-butyl peroxide) propane, 2,2-bis-(peroxy) to the mixture obtained in step S1 Oxygenated tert-butyl) butane or peracetic acid), wherein, the addition of the peroxide is set as follows: the mol ratio scope of the peroxide and the metal ion in the...
Embodiment 1
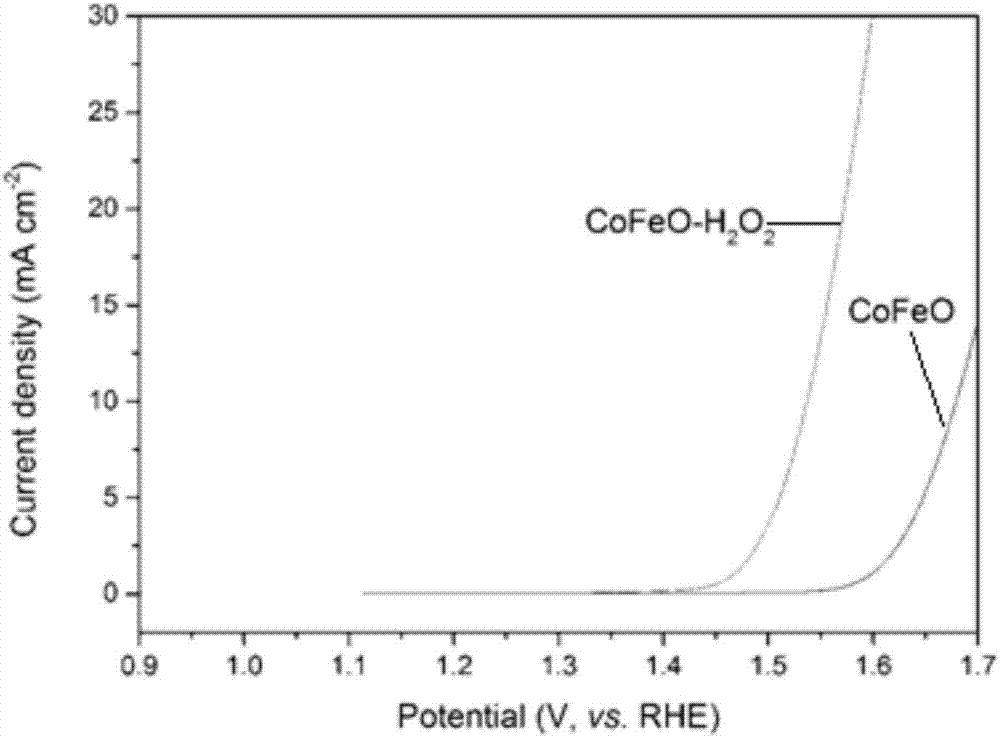
[0037] Disperse a composite oxide containing two transition metal elements Co and Fe (Co / Fe molar ratio 1:99-99:1) in a 0.005-0.5M NaOH solution at a mass concentration of 100g / L, sonicate for 1h, and then Add hydrogen peroxide, add hydrogen peroxide according to the ratio of peroxide:metal ion molar ratio of 50:1, heat to 100°C, keep warm for 5h, cool, and centrifuge the obtained mixture, the obtained solid is the high-efficiency oxygen evolution reaction Catalyst (such as figure 2 ). This chemical oxidation treatment can form oxyhydroxides with high catalytic activity of oxygen evolution reaction on the surface of the material. The potential is significantly reduced, the initial reaction potential is significantly negative compared with that before oxidation treatment, and the reaction current at 1.5V is increased by dozens of times (such as image 3 ).
Embodiment 2
[0039] Disperse the sulfide containing two kinds of transition metal elements Ni and Fe (Ni / Fe molar ratio is 1:99-99:1) in water at a mass concentration of 10g / L, stir evenly, then add peracetic acid, press Oxide: metal ion molar ratio is 5:1, heat it up to 160°C with sealing, keep it warm for 1h, filter the mixture after cooling, and the obtained solid is the high-efficiency oxygen evolution reaction catalyst.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com