Preparation method and application of visible-light-responsive Bi3O4Cl/g-C3N4 heterojunction material
A visible light, heterojunction technology, applied in chemical instruments and methods, light water/sewage treatment, water/sludge/sewage treatment, etc., can solve the problem of low photocatalytic activity, limited absorption range, unsatisfactory photocatalytic activity, etc. problem, to achieve the effect of simple process, easy mass production and excellent photocatalytic activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
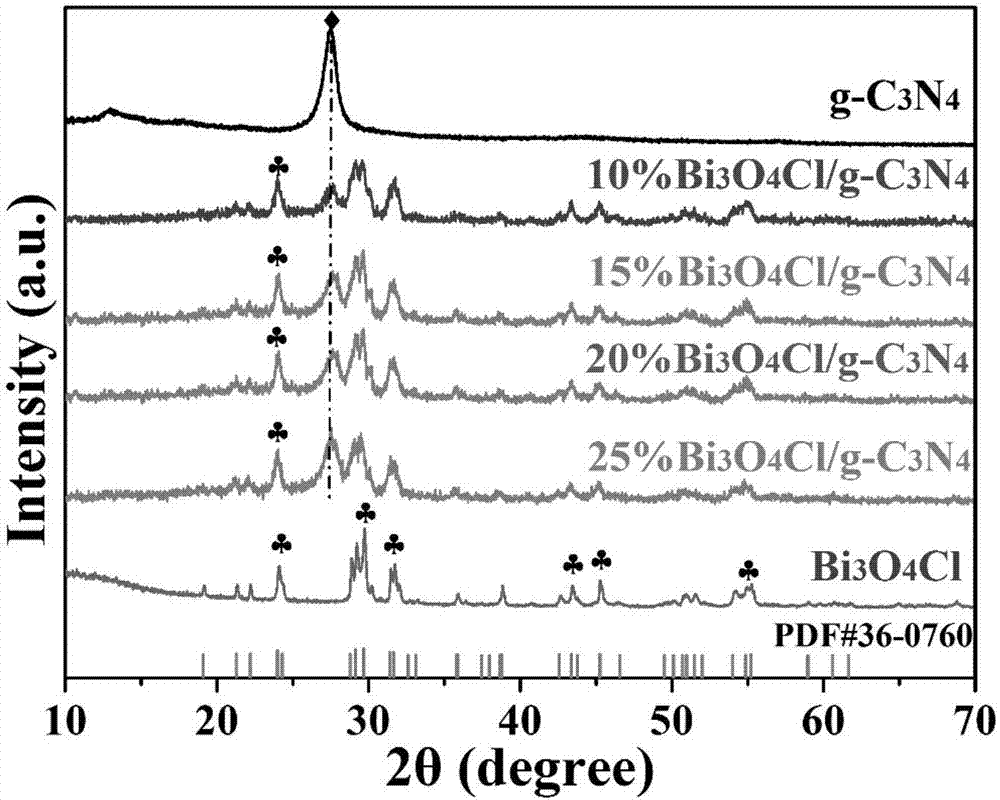
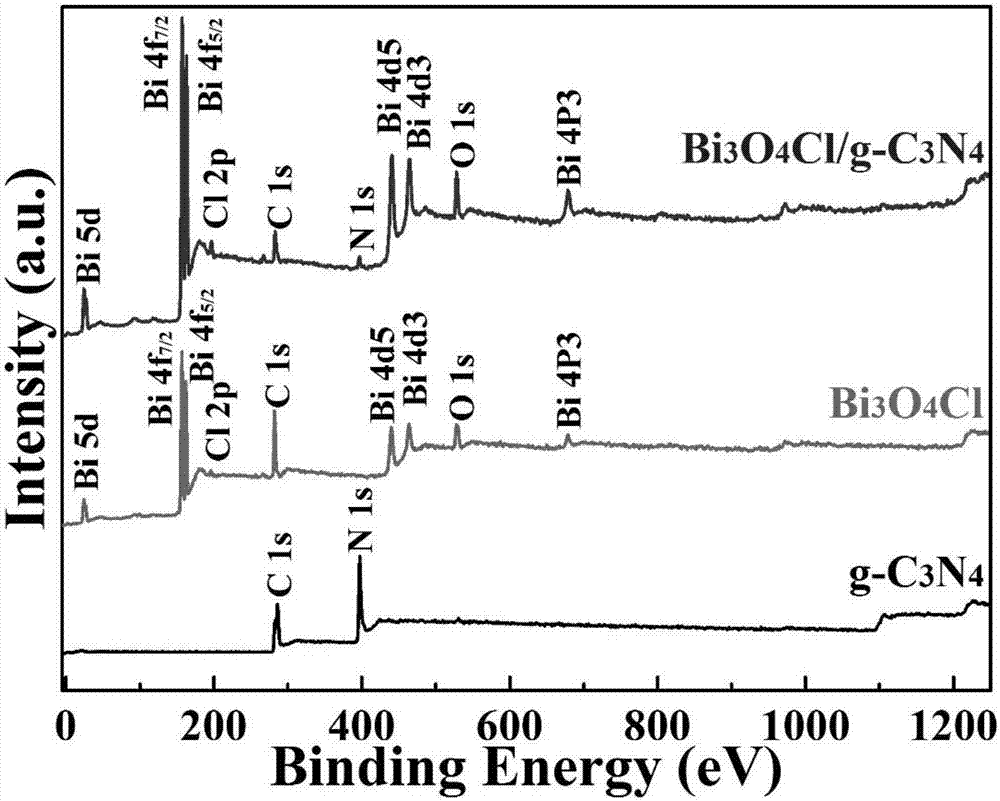
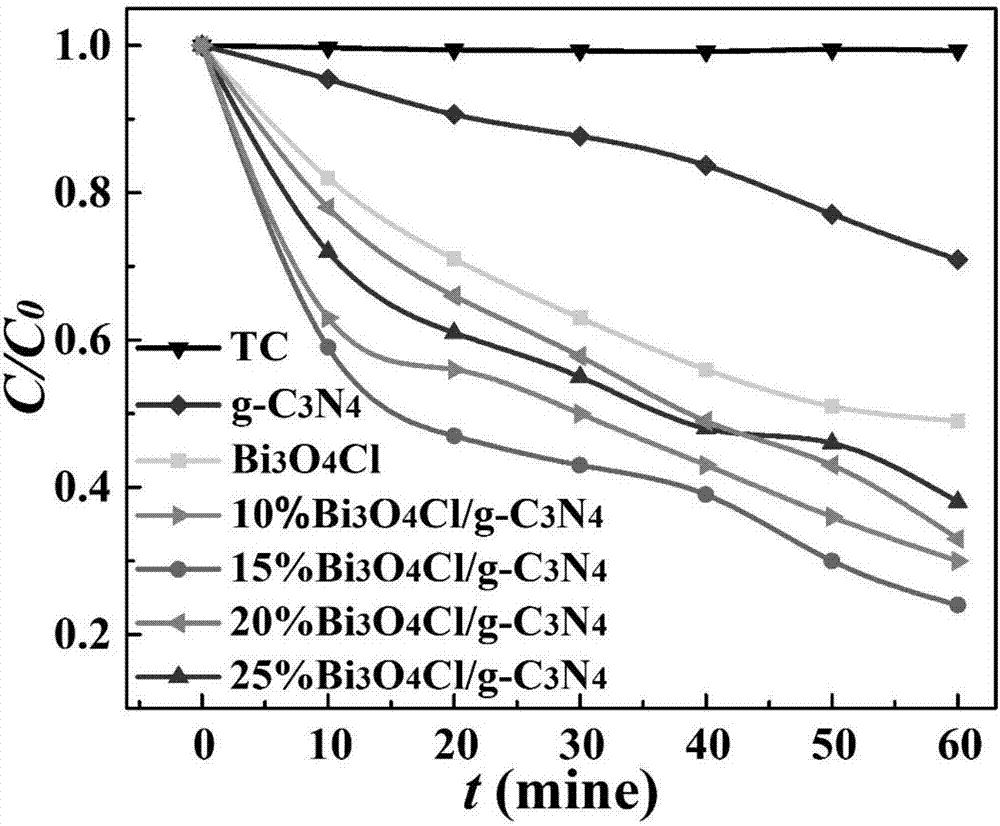
[0023] A visible light responsive Bi 3 o 4 Cl / g-C 3 N 4 A method for preparing a heterojunction material, comprising the following steps:
[0024] Step 1) Dry the urea in an oven, put it into a crucible after grinding, heat up for the first time and calcine, put it into a nitric acid solution after calcination, wash and dry it with water after stirring, put it into the crucible again after grinding, and heat up for the second time Calcined to finally get flake g-C 3 N 4 ;
[0025] Step 2) Bi(NO 3 ) 3 ·5H 2 O is ultrasonically dispersed in ethylene glycol, and solution A is obtained after stirring ultrasonically; the NH4 Cl was dissolved in deionized water to obtain solution B, and solution B was slowly added to the above solution A to form a white turbid liquid, which was transferred to the reaction kettle for hydrothermal reaction, and the reaction kettle was cooled to room temperature, washed with water and ethanol, and dried to obtain Solid powder C; finally, put t...
Embodiment 1
[0031] Step 1: Put 10g of urea in an oven at 80°C to dry for 24 hours, put it into a crucible after grinding, and calcinate at 550°C for 4 hours with a heating rate of 2°C / min. After calcination, put it into 1mol / L nitric acid solution, stir for 24h, wash and dry with water, grind again and put it into a crucible, and calcinate at 500°C for 4h with a heating rate of 5°C / min. End up with flaky g-C 3 N 4 .
[0032] Step 2: First, 0.485g Bi(NO 3 ) 3 ·5H 2 O was ultrasonically dispersed in 10 mL of ethylene glycol and stirred for 10 min to obtain solution A.
[0033] Next, add 0.018g NH 4 Dissolve Cl in 25mL deionized water to obtain solution B. Slowly add solution B to the above solution A to form a white turbid solution, transfer it to a 50mL reaction kettle, and heat at 160°C for 12h. After the reactor was cooled to room temperature, the sample was washed with water and ethanol, and dried to obtain solid powder C.
Embodiment 2
[0037] Step 1: Put 10g of urea in an oven at 80°C to dry for 24 hours, put it into a crucible after grinding, and calcinate at 550°C for 4 hours with a heating rate of 2°C / min. After calcination, put it into 1mol / L nitric acid solution, stir for 24h, wash and dry with water, grind again and put it into a crucible, and calcinate at 500°C for 4h with a heating rate of 5°C / min. End up with flaky g-C 3 N 4 .
[0038] Step 2: First, 0.485g Bi(NO 3 ) 3 ·5H 2 O was ultrasonically dispersed in 10 mL of ethylene glycol and stirred for 10 min to obtain solution A.
[0039] Next, add 0.018g NH 4 Dissolve Cl in 25mL deionized water to obtain solution B. Slowly add solution B to the above solution A to form a white turbid solution, transfer it to a 50mL reaction kettle, and heat at 160°C for 12h. After the reactor was cooled to room temperature, the sample was washed with water and ethanol, and dried to obtain solid powder C.
[0040] Finally, the solid powder C was calcined in a m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com