A new class of compounds with 5-oxime ester b2a structure and their preparation methods and applications
A compound and reaction technology, applied in the field of compounds with 5-oxime ester B2a structure, can solve the problems of low application value, waste of resources, environmental pollution and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
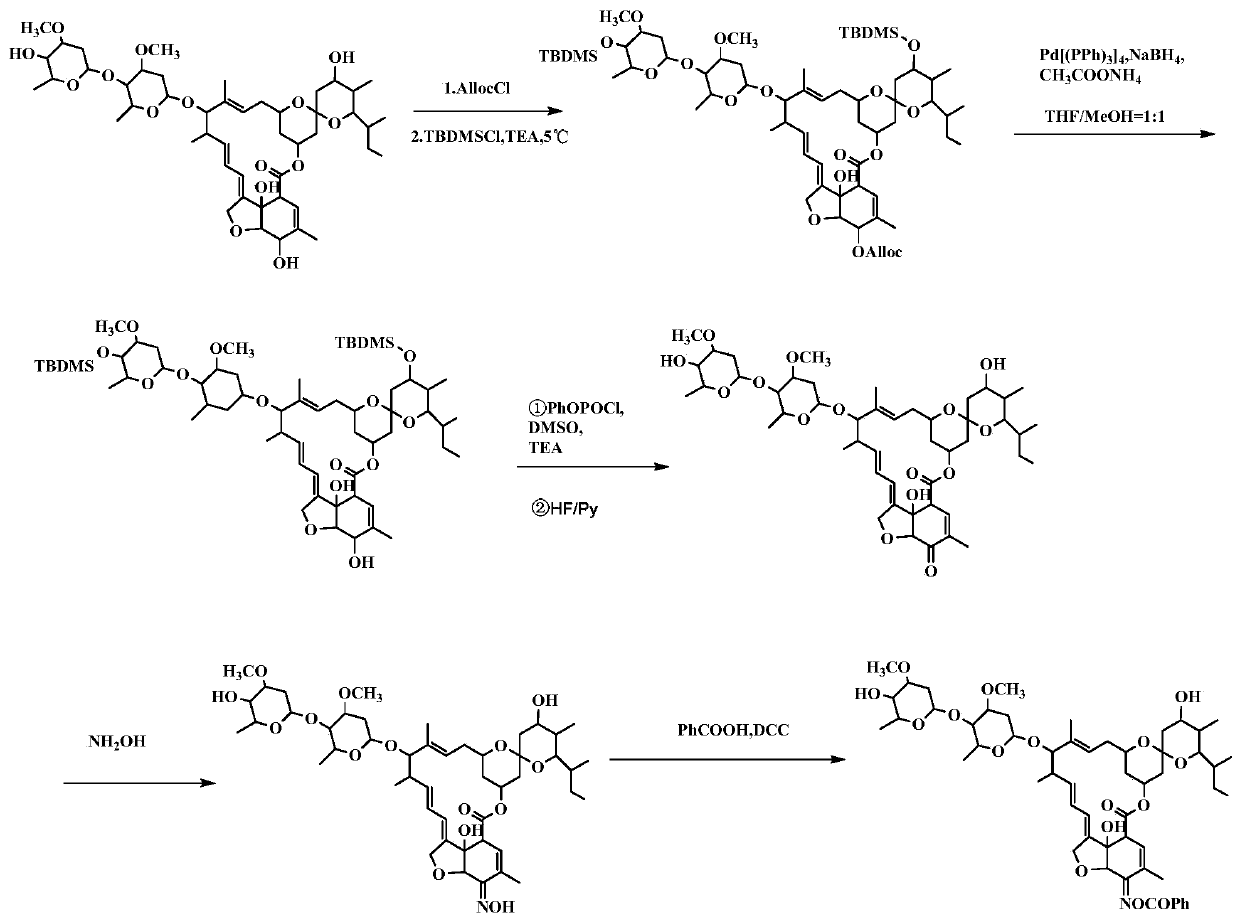
[0092] Example 1. Preparation and structure identification of compound CAU-AVM-01 (R=phenyl in formula I)
[0093] according to figure 1 The synthetic route shown is the synthesis of compound CAU-AVM-01.
[0094] (1) Add B2a (6g, 6.7mmol) in a 250ml round bottom flask, dissolve in 45ml of anhydrous dichloromethane, then add triethylamine (1.0g, 10mmol), and slowly Add allyl chloroformate (0.97g, 8mmol) dropwise, continue the reaction for 40min after the drop, TLC monitors that the reaction is complete, add 1ml of methanol to quench, extract with dichloromethane, wash with 1N hydrochloric acid, saturated sodium chloride solution, and water successively The organic phase was dried over anhydrous sodium sulfate, dichloromethane was distilled off, and purified by column chromatography to obtain 6.1 g of light yellow solid 5-O-Alloc avermectin B2a with a yield of 93%. Dissolve 5-O-Alloc avermectin B2a (6.0g, 6.2mmol) in 40ml DMF, add triethylamine (4.4g, 43mmol), place the reacti...
Embodiment 2
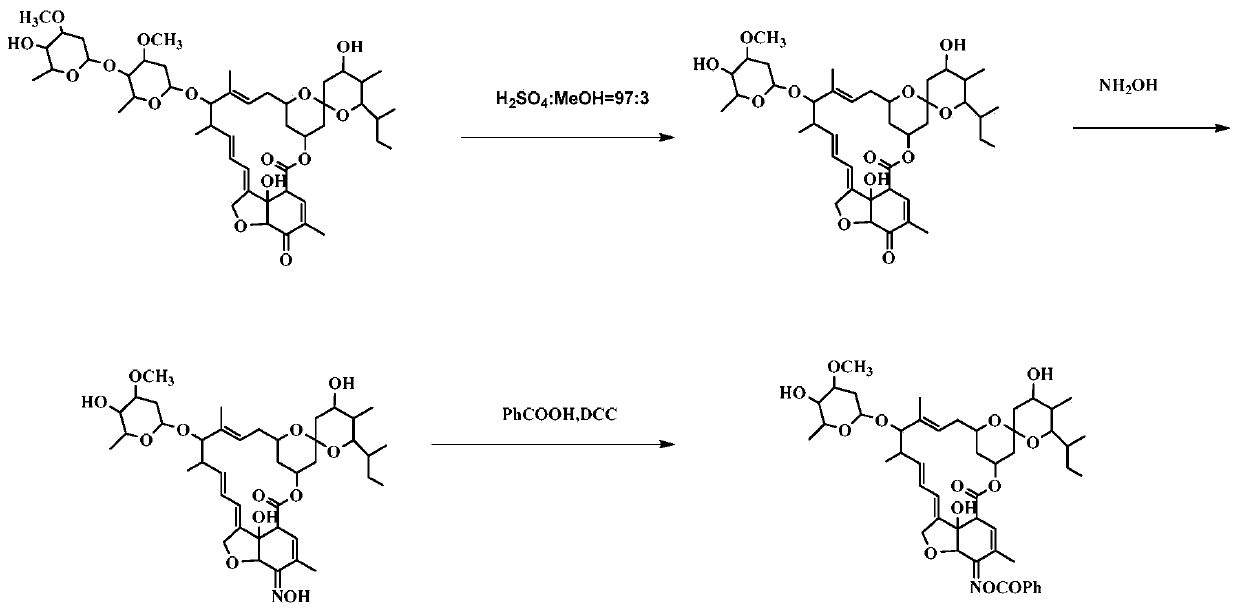
[0103] Example 2. Preparation and structural identification of compound CAU-AVM-18 (R=phenyl in formula II)
[0104] according to figure 2 The synthetic route shown shows the synthesis of compound CAU-AVM-18.
[0105] (1) Dissolve 5-O avermectin B2a (4g, 3.6mmol) in 3% sulfuric acid methanol solution, react at -5°C for 24h, neutralize with triethylamine, concentrate, extract with dichloromethane, anhydrous sodium sulfate After drying, filtering and concentrating, column chromatography gave 2.3 g of light yellow solid 5-O avermectin B2a monosaccharide, with a yield of 85%.
[0106] (2) Dissolve 5-O avermectin B2a monosaccharide (1.7g, 2.3mmol) in 10ml MeOH and 10ml 1,4-dioxane solution; dissolve hydroxylamine hydrochloride (0.94g, 13mmol) in 8ml water, and then It was added dropwise into the reaction system, TLC detected that the reaction was complete, concentrated, diluted with dichloromethane, washed with water, the aqueous layer was extracted twice with dichloromethane, t...
Embodiment 3
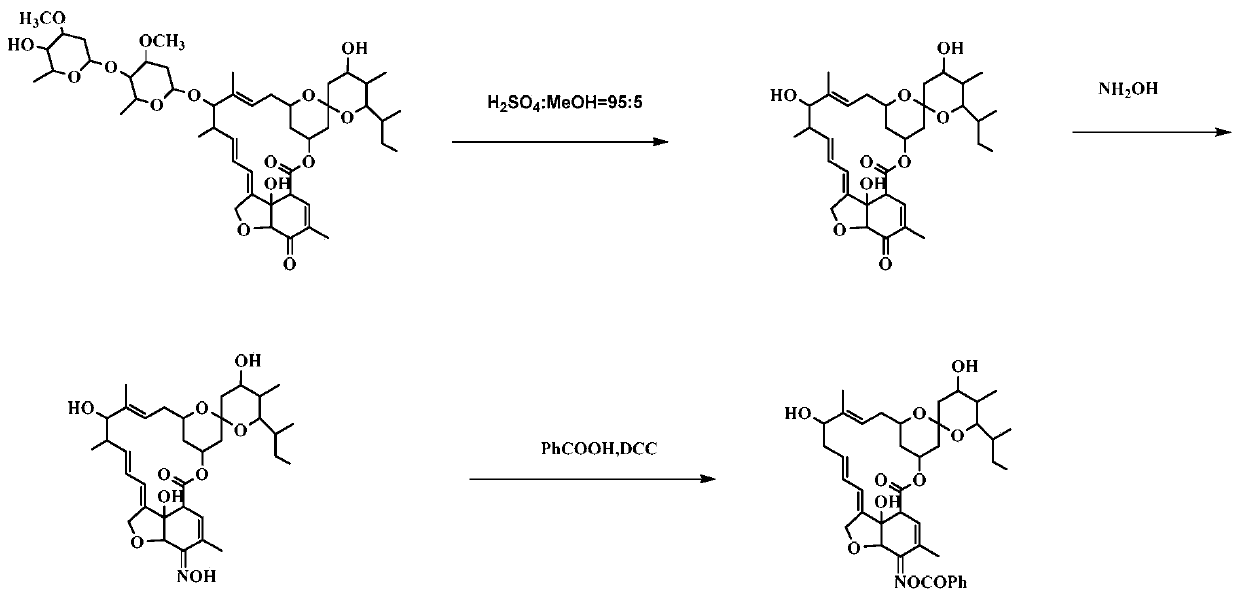
[0112] Example 3, Preparation and Structure Identification of Compound CAU-AVM-35 (R=Phenyl in Formula III)
[0113] according to image 3 The synthetic route shown is the synthesis of compound CAU-AVM-35.
[0114] (1) Dissolve 5-O avermectin B2a (4g, 3.6mmol) in 5% sulfuric acid methanol solution, react at -5°C for 24h, neutralize with triethylamine, concentrate, extract with dichloromethane, anhydrous sodium sulfate After drying, filtering and concentrating, column chromatography gave 2.0 g of light yellow solid 5-O avermectin B2a aglycone with a yield of 93%.
[0115] (2) Dissolve 5-O avermectin B2a aglycone (1.4g, 2.3mmol) in 10ml MeOH and 10ml 1,4-dioxane solution; dissolve hydroxylamine hydrochloride (0.94g, 13mmol) in 8ml water, and then It was added dropwise into the reaction system, TLC detected that the reaction was complete, concentrated, diluted with dichloromethane, washed with water, the aqueous layer was extracted twice with dichloromethane, the organic phase ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com