Continuous determination method for contents of sulfuric acid, copper and nickel in copper electrolyte
A technology of copper electrolyte and measurement method, which is applied in the direction of chemical analysis by titration method, etc., can solve the problems of large dilution factor analysis result error, large instrument loss, high concentration of salt and sulfuric acid, etc., to save analysis and detection time and save labor The effect of cost and result stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment example 1
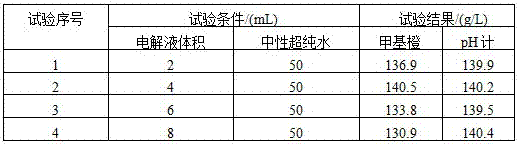
[0031] A method for continuous determination of sulfuric acid, copper, and nickel content in copper electrolyte. Add 70 mL of neutral ultrapure water to the same sample of copper electrolyte with a volume of 10 mL, use a pH meter to indicate the end point, and use acid-base titration Determination of sulfuric acid by the method; after measuring the sample of sulfuric acid, adjust the pH to 4.0 with ammonia water, add ammonium bifluoride, potassium iodide, and starch indicator, and use sodium thiosulfate titration to determine the copper in the copper electrolyte; after measuring the copper Add 7mL of tartaric acid solution to the sample, adjust the pH to 9.0 with ammonia water, add 14mL of sodium thiosulfate solution and ammonium violin indicator, titrate with EDTA standard solution, and measure the nickel content in the electrolyte.
[0032] The alkali in the described acid-base titration method is sodium hydroxide standard solution.
[0033] The neutral ultrapure water refer...
Embodiment example 2
[0040] A method for continuous determination of sulfuric acid, copper and nickel content in copper electrolyte. Add 30mL of neutral ultrapure water to the same sample of copper electrolyte with a volume of 2mL, use a pH meter to indicate the end point, and use acid-base titration Determination of sulfuric acid by the sulfuric acid method; in the sample of sulfuric acid, adjust the pH to 3.0 with ammonia water, add ammonium bifluoride, potassium iodide, starch indicator, and use sodium thiosulfate titration to determine the copper in the copper electrolyte; after measuring the copper Add 3mL tartaric acid solution to the sample, adjust the pH to 8.0 with ammonia water, add 6mL sodium thiosulfate solution and ammonium violin indicator, titrate with EDTA standard solution, and measure the nickel content in the electrolyte.
[0041] The alkali in the described acid-base titration method is sodium hydroxide standard solution.
[0042] The neutral ultrapure water refers to the ultra...
Embodiment example 3
[0049] A method for continuous determination of sulfuric acid, copper, and nickel content in copper electrolyte. Add 50 mL of neutral ultrapure water to the same sample of copper electrolyte with a volume of 6 mL, use a pH meter to indicate the end point, and use acid-base titration Determination of sulfuric acid by the method; in the test sample of sulfuric acid, adjust the pH to 3.5 with ammonia water, add ammonium bifluoride, potassium iodide, starch indicator, and use sodium thiosulfate titration to determine the copper in the copper electrolyte; after measuring the copper Add 3-7mL tartaric acid solution to the sample, adjust the pH to 8.5 with ammonia water, add 10mL sodium thiosulfate solution and ammonium violin indicator, titrate with EDTA standard solution, and measure the nickel content in the electrolyte.
[0050] The alkali in the described acid-base titration method is sodium hydroxide standard solution.
[0051] The neutral ultrapure water refers to the ultrapur...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com