Application of MgIG (magnesium isoglycyrrhizinate) in preparing treatment drug for relieving renal injury caused by aminoglycoside antibiotics
A technology of aminoglycosides and magnesium isoglycyrrhizinate, applied in the field of natural medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0013] Example 1: Determination of renal function-related biochemical indicators in SD rats after multiple administrations
[0014] The main steps are as follows:
[0015] Thirty-six male SD rats were randomly divided into six groups according to body weight, with 6 rats in each group.
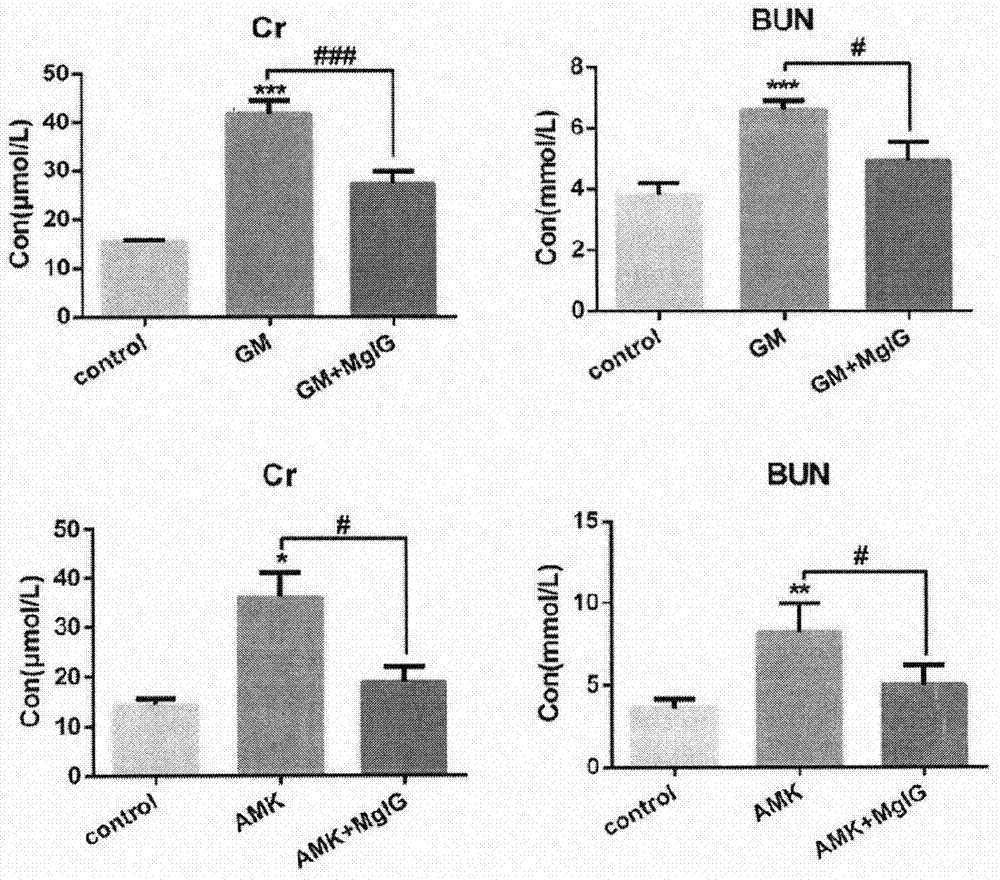
[0016] 1. Protective effect of magnesium isoglycyrrhizinate on gentamicin-induced renal injury model: gentamicin combined with magnesium isoglycyrrhizinate medication group: SD rats were pre-administered magnesium isoglycyrrhizinate for 5 days (i.p 50mg / kg / day), starting from the 6th day, magnesium isoglycyrrhizinate was given Half an hour after the medicine, 70 mg / kg gentamicin was administered intraperitoneally (i.p.) for 7 consecutive days. Gentamicin alone group: During the pre-administration period of magnesium isoglycyrrhizinate, an equal volume of normal saline was injected intraperitoneally every day, and from the 6th day, 70 mg / kg gentamicin was administered intraperitoneally (i.p.) ...
Embodiment 2
[0020] Embodiment 2: Kidney histopathological examination after repeated administration of SD rats
[0021] The main steps are as follows:
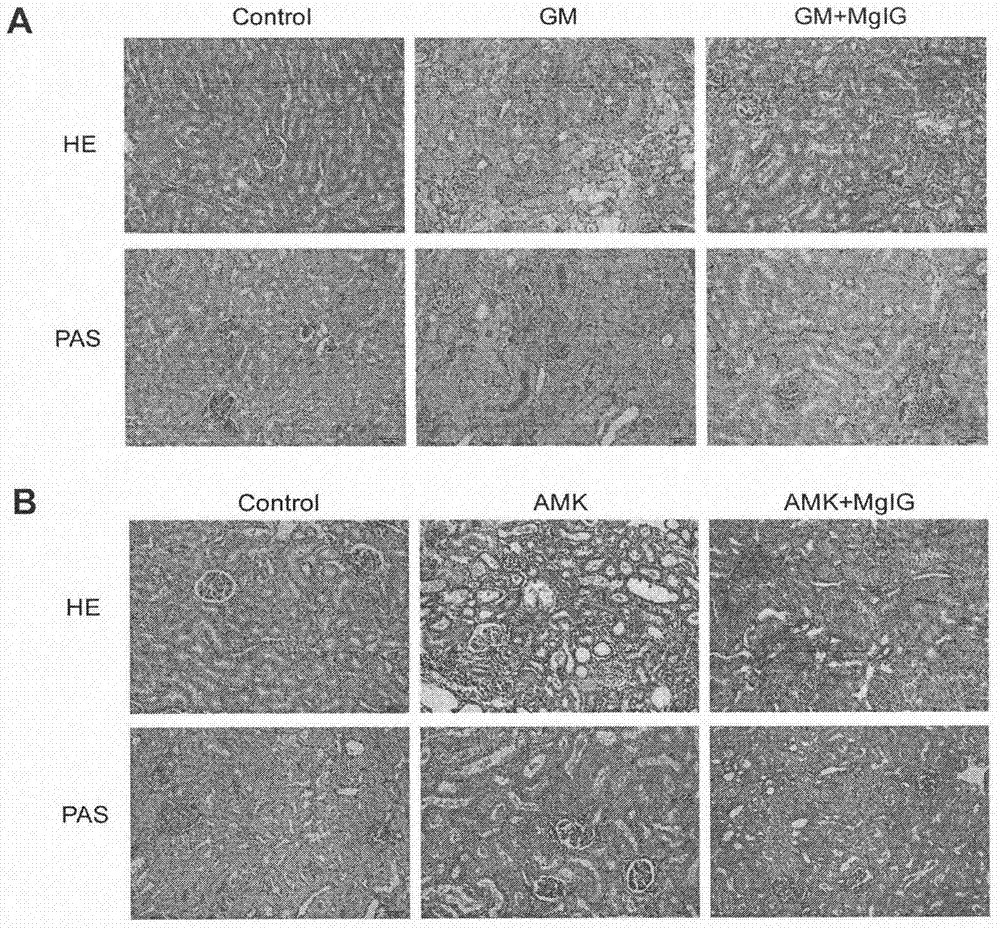
[0022] The aminoglycoside drug-induced kidney injury model establishment method and dosage regimen are the same as in Example 1. The rats were sacrificed 4 hours after administration on the last day, and the left kidney was cut longitudinally, half of which was fixed with 4% paraformaldehyde for histopathological detection (HE / PAS staining).
[0023] Experimental results:
[0024] Pathological examination results such as figure 2shown. In the gentamicin (GM)-induced renal injury model group and the amikacin (AMK)-induced renal injury model group, tubular necrosis in the renal cortex area, dilated renal tubules, increased basophilic renal tubules, interstitial Inflammatory cell infiltration was more severe than that in the blank control group; under the PAS staining microscope, the brush border of the renal proximal tubule was obvious...
Embodiment 3
[0025] Example 3: Expression of mRNA levels of mitochondrial bioremediation regulatory factors in kidney tissue of SD rats after multiple administrations
[0026] The main steps are as follows:
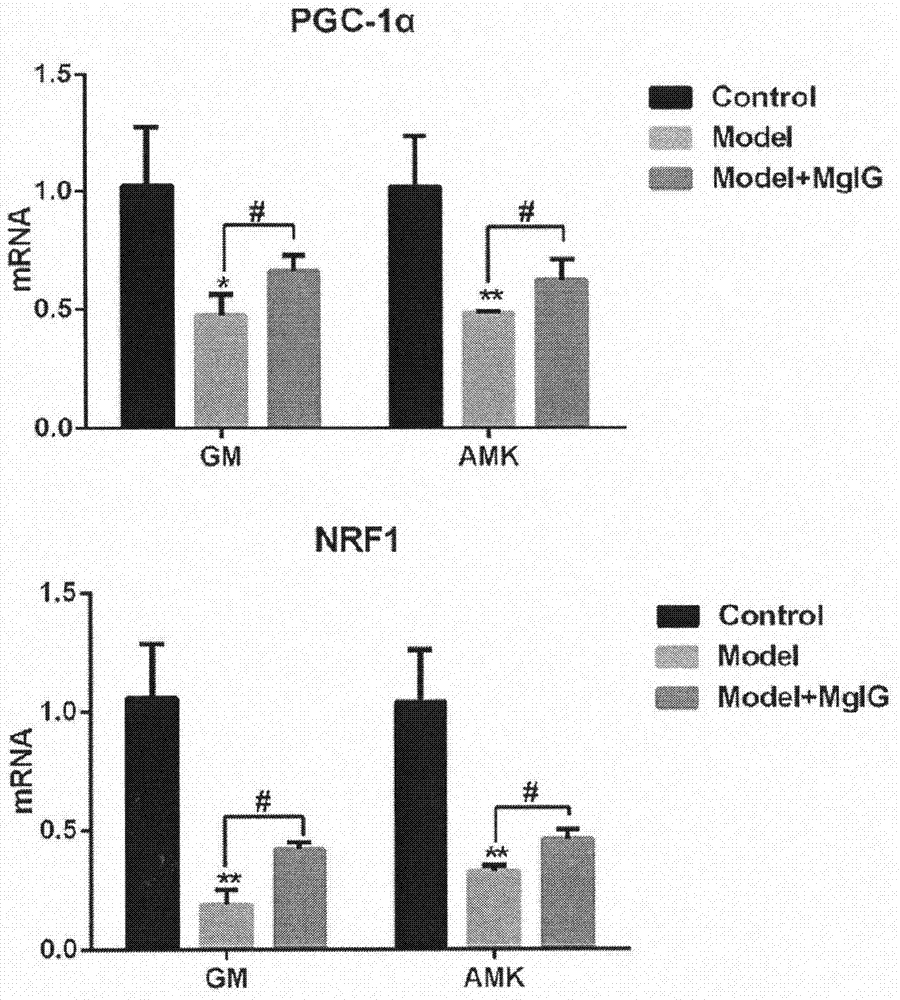
[0027] The aminoglycoside drug-induced kidney injury model establishment method and dosage regimen are the same as in Example 1. The rats were sacrificed 4 hours after administration on the last day, and half of the left kidney was cut longitudinally. Kidney tissue RNA was extracted according to the Takara total RNA extraction kit, and the RNA was quantified. According to the instructions of the RT-PCR kit, the mRNA was reverse transcribed into cDNA. Real-time fluorescent quantitative PCR analysis was performed according to the instructions of Thermal Cycler DiceTM RealTime System (TakaRa Code: TP800). The expression of mRNA levels of mitochondrial biorepair regulators in kidney tissue: peroxisome proliferator-activated receptor gamma coactivator 1α (PGC-1α) and nuclear respiratory...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com