Acid addition salt of deuteration dehydrogenation phenyl plinabulin compound and application thereof to anti-tumor medicine preparation
A technology of phenylahistine and acid addition salt, which is applied in the field of medicinal chemistry, can solve the problems of unreported preparation methods, etc., and achieve the effects of improving bioavailability, broadening dosage form selection, and improving solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] The preparation method of the deuterated dehydrophenylahistine compound having the structure shown in the general formula (I) of the present invention comprises the following steps:
[0032] (1) Synthesis of deuterium aldehyde compound b: starting from the 5-tert-butyl-1H-imidazole-4-carbaldehyde, via NaBD 4 Reduction and manganese dioxide oxidation afford 5-tert-butyl-1H-imidazole-4-deuterocarbaldehyde,
[0033]
[0034] (2) The first step of condensation reaction: first condensing diacetylpiperazine diketone (DKP) with the aldehyde intermediate a or the deuterium aldehyde compound b to form a heterocyclic compound c or a deuterium-containing heterocyclic compound d;
[0035]
[0036] (3) The second condensation reaction: the heterocyclic compound c or the deuterium-containing heterocyclic compound d is condensed with the second condensation reaction aldehyde to form the deuterated dehydrophenylahistin compound; The second condensation reaction aldehyde is benza...
Embodiment 2
[0039] ((3Z,6Z)-3-Benzylidene-6-((5-tert-butyl-1H-imidazol-4-yl)deuteromethylene)piperazine-2,5-dione hydrochloride preparation of
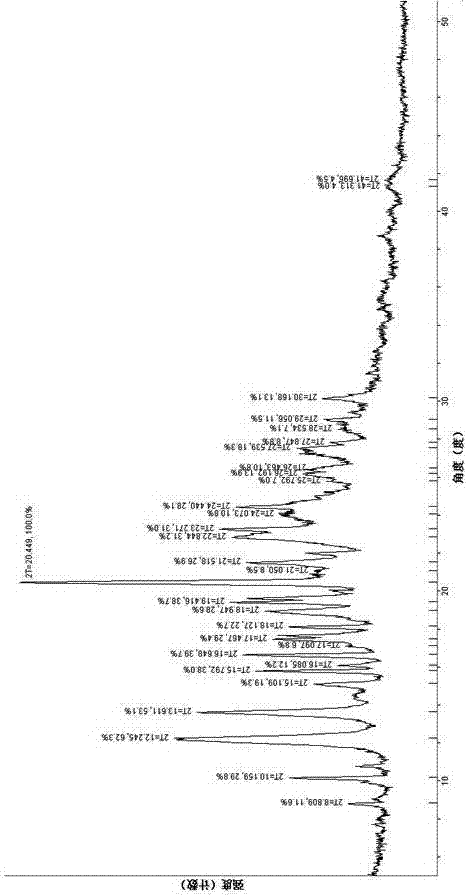
[0040]Its specific preparation process includes the following steps: take ((3Z,6Z)-3-benzylidene-6-((5-tert-butyl-1H-imidazol-4-yl) deuterated methylene) piperazine- 2,5-diketone (100mg, 0.30mmol) was dissolved in 3ml of acetone, and hydrochloric acid (16mg, 0.44mmol) diluted with acetone was added dropwise. The reaction was stirred at room temperature for 1.5h, suction filtered, and the filter cake was washed with acetone to obtain a white solid, which was mixed with Hydrochloric acid was salified at a molar ratio of 1:1, and the yield was 72%. m.p.290-291°C; 1H NMR (500MHz, DMSO-d6) δ13.07(brs,1H),11.61(brs,1H),10.19(s ,1H),8.36(brs,1H),7.51(d,J=7.7Hz,2H),7.41(t,J=7.6Hz,2H),7.31(t,J=7.3Hz,1H),6.77(s ,1H),1.35(s,9H), tested by X-ray powder diffraction, the specific characteristic absorption peaks are shown in figure 1 .
Embodiment 3
[0042] ((3Z,6Z)-3-Benzylidene-6-((5-tert-butyl-1H-imidazol-4-yl)deuteromethylene)piperazine-2,5-dionemethanesulfonic acid salt preparation
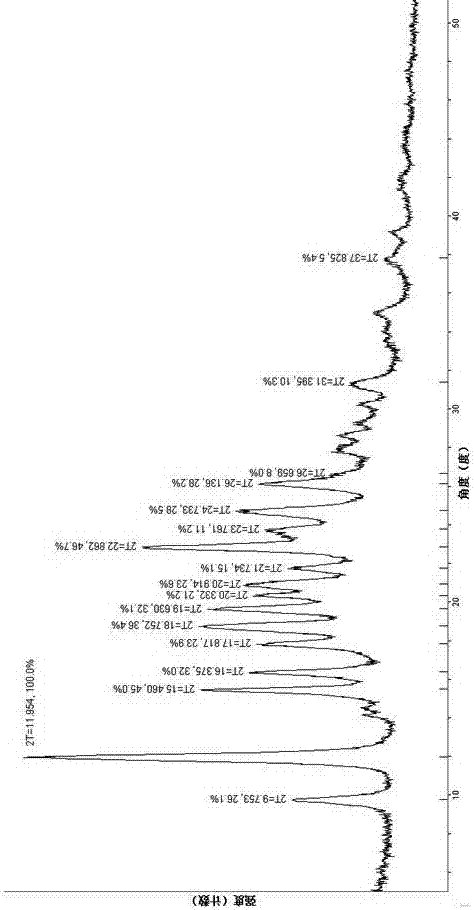
[0043] Its specific preparation process includes the following steps: take ((3Z,6Z)-3-benzylidene-6-((5-tert-butyl-1H-imidazol-4-yl) deuterated methylene) piperazine- 2,5-Diketone (200mg, 0.59mmol) was dissolved in 4ml of acetone at room temperature, and methanesulfonic acid (86mg, 0.89mmol) diluted with acetone was added dropwise. The reaction was stirred at room temperature for 1.5h, suction filtered, and the filter cake was washed with acetone. 229 mg of white solid was obtained, the yield was 89%, and it was formed into a salt with methanesulfonic acid at a molar ratio of 1:1. m.p. ,1H),10.20(s,1H),8.31(brs,1H),7.53(d,J=7.6Hz,2H),7.43(t,J=7.7Hz,2H),7.33(t,J=7.3Hz , 1H), 6.78(s, 1H), 2.33(s, 3H), 1.37(s, 9H), by X-ray powder diffraction test, the specific characteristic absorption peaks are shown in figure 2 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com