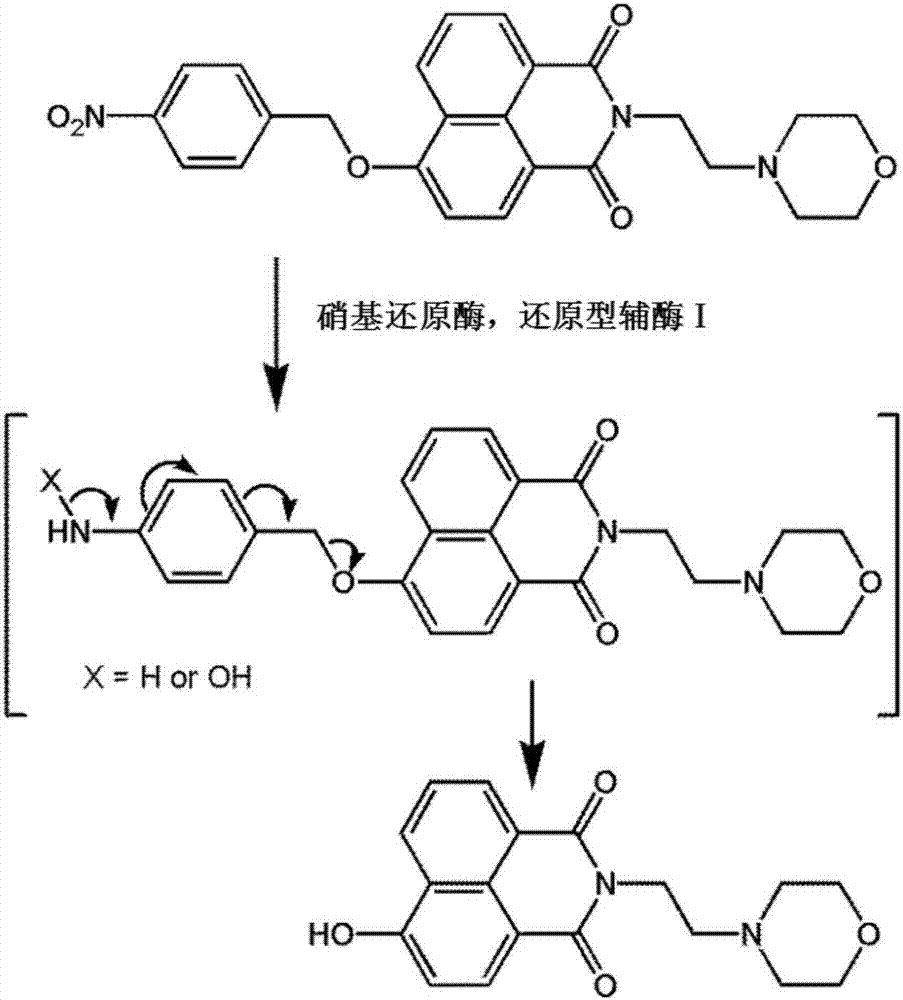
Fluorescence probe for detecting nitroreductase and application thereof
A technology of nitroreductase and fluorescent probe, applied in the field of fluorescent probe
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
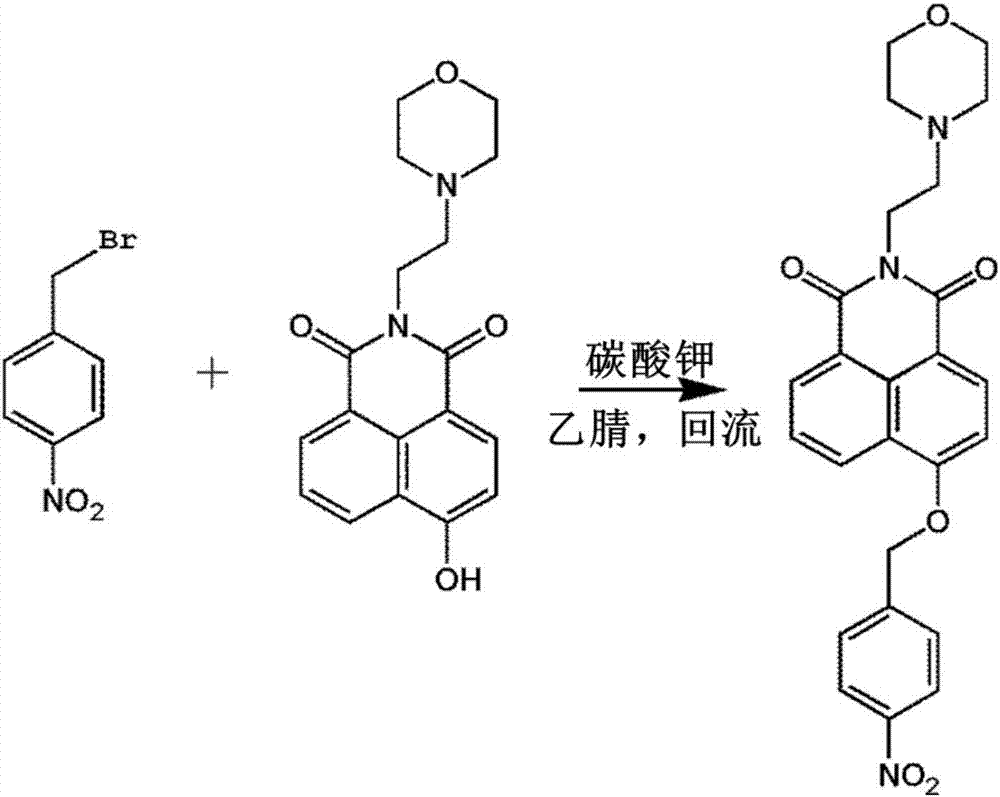
[0019] Embodiment 1 (synthesis of fluorescent probe A):
[0020] The synthesis process of fluorescent probe A is as follows: figure 2 shown. Add N-morpholinoethylamino-4-hydroxyl-1,8-naphthalimide (0.33g, 1mmol), potassium carbonate (1.00g), p-nitrobenzyl bromide (0.43g, 2mmol) into a 250mL three-necked flask ), after 3 times of vacuum / argon replacement, add 150ml of anhydrous acetonitrile, heat up to acetonitrile reflux under the protection of argon, heat and stir for 4 hours. The reaction solution was evaporated under reduced pressure, and the obtained solid was purified by column chromatography to obtain the target compound fluorescent probe A. 1 HNMR (400MHz, CDCl 3 )δ(ppm)8.63(d,J=8.0Hz,2H),8.54(d,J=8.0Hz,1H),8.33(d,J=8.4Hz,2H),7.78–7.72(m,3H), 7.10(d, J=8.0Hz, 1H), 5.49(s, 1H), 4.34(t, J=7.0Hz, 2H), 3.70(t, J=4.2Hz, 4H), 2.73(t, J=6.8 Hz,2H),2.63(s,4H). 13 CNMR(100MHz,CDCl3)δ(ppm):14.14,22.70,29.71,31.93,37.03,53.77,56.17,66.94,69.46,106.42,115.93,122.55,124.15,12...
Embodiment 2
[0021] Embodiment 2 (absorption spectrum and emission spectrum response of fluorescent probe A to nitroreductase):
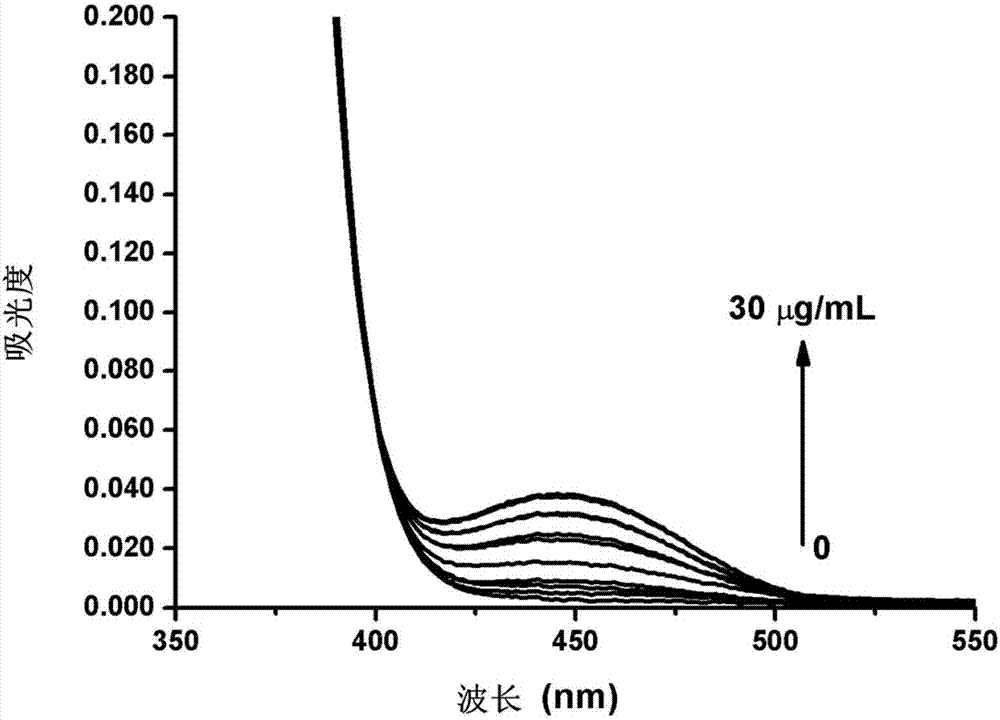
[0022] Add different volumes of nitroreductase solution (1mg / mL). After reacting at 37° C. for 60 min, the absorption spectrum and fluorescence emission spectrum of the working solution were measured, and the excitation wavelength was 445 nm.
[0023] The experimental results of the absorption spectrum and emission spectrum response of fluorescent probe A to nitroreductase are as follows: image 3 and Figure 4 shown. From image 3 It can be seen from the figure that as the amount of nitroreductase added increases (from 0 to 30 μg / mL), the absorbance of the solution system at 445 nm increases gradually, and a new absorption peak appears, which is contributed by product B. Figure 4 a shows that with the increase of the amount of nitroreductase added, the fluorescence of the system is significantly enhanced, and the new fluorescence peak at 550nm is contrib...
Embodiment 3
[0024] Embodiment 3 (kinetic experiment of nitroreductase catalyzed fluorescent probe A reaction)
[0025] Add different concentrations of fluorescent probe A to the phosphate buffer solution (pH=7.2, 10 mM) containing nitroreductase (25 μg / mL) and reduced coenzyme I (0.5 mM) volume 5%), measure the fluorescence intensity of the solution at 550 nm at intervals of 1 min at 37° C. with a microplate reader, and the excitation wavelength is 445 nm.
[0026] In addition, solutions containing different known concentrations of molecule B (synthesized according to literature) were prepared under the same conditions to obtain a standard curve representing the fluorescence intensity and concentration of molecule B at 550 nm.
[0027] The kinetic experiment results of nitroreductase catalyzing the reaction of fluorescent probe A are as follows: Figure 5 As shown, the abscissa indicates the reaction time (s), and the ordinate indicates the fluorescence intensity at 550nm, the concentrat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com