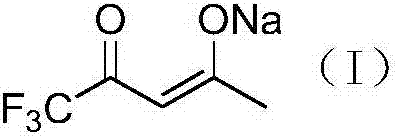
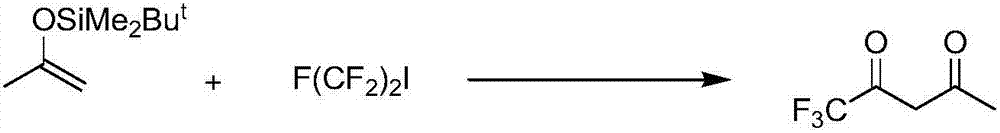Preparation method for trifluoroacetylacetone
A technology of trifluoroacetylacetone and trifluoroacetate, applied in the field of preparation of trifluoroacetylacetone, can solve the problems of complicated post-processing, unfavorable industrialization amplification, low yield and the like, and achieves mild process conditions and low production cost , the effect of high raw material yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
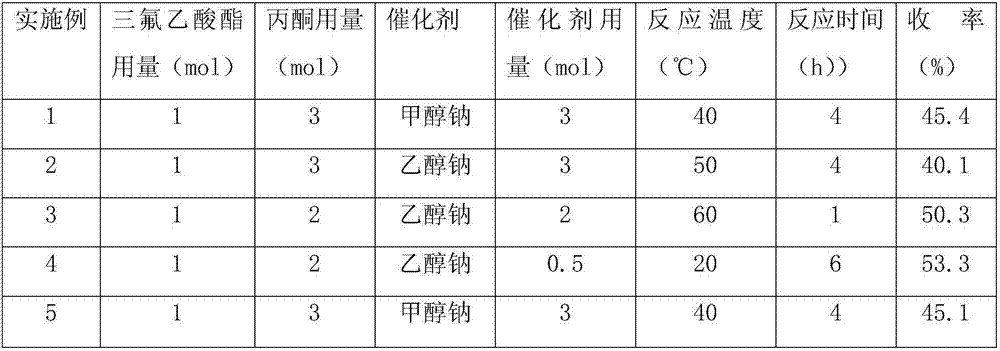
[0025] In a 1000 mL three-necked flask, add 500 mL of methanol, add 162 g (3 moles) of sodium methylate, 128 g (1 mole) of methyl trifluoroacetate and 174 g (3 moles) of acetone while stirring, and heat the reaction to 40 ° C. After 4 hours of reaction, the Under a vacuum of 0.01Mpa, heat to 40°C to distill methanol, add 294g (3mol) of sulfuric acid to the reaction solution for acidification, and distill trifluoroacetylacetone under reduced pressure under a vacuum of 0.09MPa, collect the fractions at 31 to 33°C to obtain 70.5 g of colorless liquid, GC content of trifluoroacetylacetone 99.2%, yield 45.4%.
Embodiment 2
[0027] In a 1000 mL three-necked flask, add 500 mL of ethanol, add 204 g (3 moles) of sodium ethylate, 142 g (1 mole) of ethyl trifluoroacetate and 174 g (3 moles) of acetone under stirring, and heat the reaction to 50 ° C. After 4 hours of reaction, the Under a vacuum of 0.01Mpa, heat to 50°C to distill ethanol, add 294g (3mol) sulfuric acid to the reaction solution for acidification, and distill trifluoroacetylacetone under reduced pressure under a vacuum of 0.09MPa, collect the cuts at 31 to 33°C to obtain 63.6 g of colorless liquid, GC content of trifluoroacetylacetone 99.1%, yield 40.1%.
Embodiment 3
[0029] In a 1000 mL three-neck flask, add 500 mL of toluene, add 136 g (2 moles) of sodium ethylate, 156 g (1 mole) of 4-methoxyphenyl trifluoroacetate and 116 g (2 moles) of acetone under stirring, and heat the reaction to 60 ° C , after reacting for 1 hour, under the vacuum of 0.01Mpa, be heated to 70 DEG C and distill out toluene, add 138g (3mol) formic acid acidification in the reaction liquid, under the vacuum of 0.09MPa, distill out trifluoroacetylacetone under reduced pressure, collect 31 From the fraction at ~33°C, 75.6 g of colorless liquid was obtained, the GC content of trifluoroacetylacetone was 99.2%, and the yield was 50.3%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com