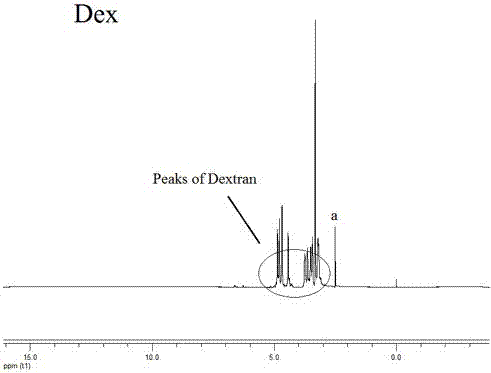
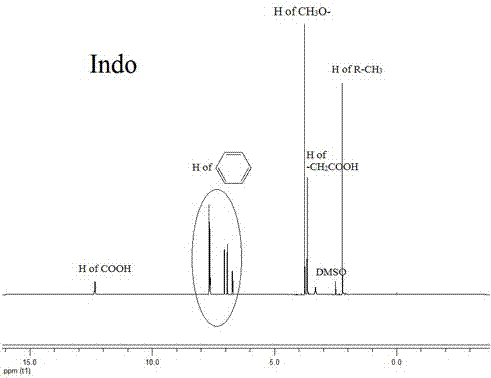
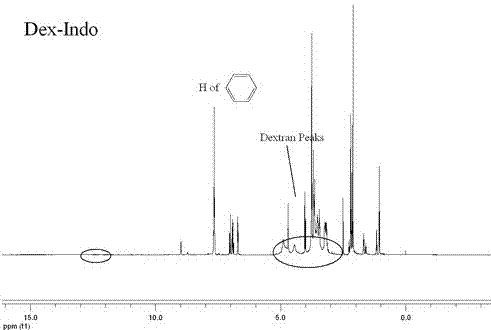
Synthetic method and application of glucan/indometacin graft
A technology of indomethacin grafts and synthesis methods, which is applied in the direction of drug combinations, active ingredients of heterocyclic compounds, medical preparations of non-active ingredients, etc., can solve problems such as high toxicity, and achieve high drug loading, tumor Increased cell inhibition rate and simple preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] Weighed indomethacin (3.086mmol, 1103mg), EDC (9.258mmol, 1774mg) and DMAP (0.9258mmol, 113.0mg), dissolved in 10mL of DMSO, stirred to dissolve completely, kept at constant temperature at 60°C for 30min, slowly Add dextran (3.086mmol, 500mg) (Mw=10000), stir and react at 25°C for 24h, then transfer the reaction solution to a dialysis bag (MWCO=7000), dialyze with double distilled water as the medium, and continue to replace the medium for 48h , after the dialysis, collect the milky liquid in the dialysis bag, at 4 DEG C, centrifuge 10min at 4000rpm, remove the insoluble matter, get the supernatant and freeze-dry to obtain the dextran / indomethacin graft (hereinafter abbreviated as Dex / Indo) freeze-dried product 1.
[0056] The obtained dextran / indomethacin graft lyophilized product 1 was Dex10000 / Indo20%.
Embodiment 2
[0058] Separately weigh indomethacin (1.543mmol, 551.5mg), EDC (4.629mmol, 886.9mg) and DMAP (0.4629mmol, 56.0mg), dissolve in 10mL DMSO, stir to dissolve completely, and keep at constant temperature 70°C for 20min , slowly added dextran (3.086mmol, 500mg) (Mw=10000), stirred and reacted at 70°C for 12h, then transferred the reaction solution to a dialysis bag (MWCO=5000), dialyzed with double distilled water as the medium, and continuously replaced Medium 48h, after dialysis finishes, collect the milky liquid in the dialysis bag, at 10 ℃, 2000rpm centrifugal 50min, remove insoluble matter, get the supernatant and freeze-dry to obtain dextran / indomethacin graft (hereinafter abbreviated as Dex / Indo) freeze-dried product 2.
[0059] The obtained dextran / indomethacin graft lyophilized product 2 was Dex10000 / Indo50%.
Embodiment 3
[0061] Separately weigh indomethacin (0.617mmol, 220.6mg), EDC (1.851mmol, 354.7mg) and DMAP (0.1851mmol, 23.0mg), dissolve in 10mL DMSO, stir to dissolve completely, keep at constant temperature 20°C for 12h , slowly added dextran (3.086mmol, 500mg) (Mw=10000), stirred and reacted at 20°C for 48h, then transferred the reaction solution to a dialysis bag (MWCO=6000), dialyzed with double distilled water as the medium, and continuously replaced Medium 48h, after dialysis finishes, collect the milky liquid in the dialysis bag, at 2 ℃, 8000rpm centrifugal 20min, remove insoluble matter, get the supernatant liquid freeze-drying and promptly obtain dextran / indomethacin graft (hereinafter abbreviated as Dex / Indo) freeze-dried product3.
[0062] The obtained dextran / indomethacin graft lyophilized product 3 was Dex10000 / Indo100%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com