Pyriproxyfen synthesis method
A synthesis method and a technology for disopyramide, which are applied in the field of synthesis of disopyr ether, can solve the problems of difficult industrialized production, cumbersome methods and the like, and achieve the effects of easy industrialized production and simple process method.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
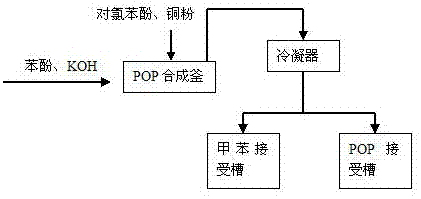
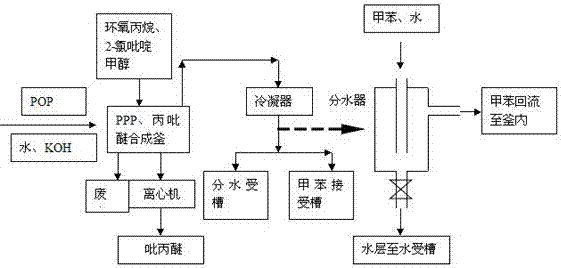
[0024] Such as figure 1 and figure 2 As shown, the specific production process steps are as follows:
[0025] 1. Synthesis of POP
[0026] Put 1600Kg of toluene, 440Kg of phenol, and 275KG of potassium hydroxide into the reaction kettle in turn, raise the temperature, and bring water under reflux until the temperature of the kettle reaches 110°C and bring it to anhydrous; evaporate the toluene, and add 10Kg of catalyst copper powder into the reaction kettle at one time During heating, at 140°C, 600Kg of p-chlorophenol was added dropwise, the dropping temperature was controlled at 140-150°C, and the dropping time was about 4 hours. Phenol < 1%, the reaction is over; use vacuum distillation to distill out POP: 782Kg (content 98%, yield: 90%, based on p-chlorophenol);
[0027] 2. Synthesis of PPP
[0028] Put 2000Kg of water, 247Kg of potassium hydroxide, and 782Kg of POP into the reaction kettle, start stirring, and drop 240Kg of propylene oxide at 10-20°C for about 3 hours...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com