Method for removing iron from low-nickel sulfonium nitric acid leaching solution through pyrolysis
A technology of leachate and ferric nitrate, applied in the field of iron removal from nickel-cobalt nitrate solution and iron removal from nitrate solution, can solve the problems of high iron removal cost and low metal recovery rate, and achieve low cost, nickel-cobalt The recovery rate is high, which is beneficial to the effect of subsequent purification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0011] The composition of a low-nickel sulfur nitric acid leaching solution is shown in Table 1.
[0012] Table 1 Low nickel sulfonium nitric acid leaching solution composition (g / L)
[0013]
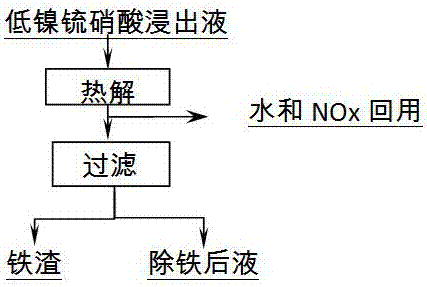
[0014] Measure 4L of the above solution, heat it to 100~150℃, react for 1~5h, pyrolyze to produce iron slag, NOx and water are recovered and recycled. After the reaction is completed and filtered, the filter residue is hydrolyzed iron slag, and the filtrate is the liquid after iron removal.
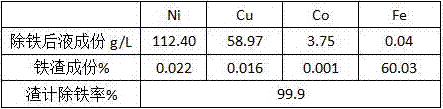
[0015] Test results: 397.4g of iron slag was produced, and 1985ml of liquid after iron removal. Table 2 shows the liquid composition, iron slag composition and slag-based iron removal rate after iron removal.
[0016] Table 2 Iron removal results
[0017]
Embodiment 2
[0019] The composition of a low-nickel sulfur nitric acid leaching solution is shown in Table 3.
[0020] Table 3 Low nickel sulfonium nitric acid leaching solution composition (g / L)
[0021]
[0022] Measure 8L of the above solution, heat it to 100~150℃, react for 1~5h, pyrolyze to produce iron slag, NOx and water are recovered and recycled. After the reaction is completed and filtered, the filter residue is hydrolyzed iron slag, and the filtrate is the liquid after iron removal.
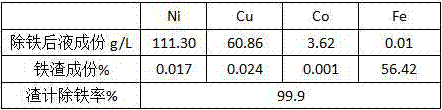
[0023] Test results: 756.3g of iron slag and 4280ml of solution were produced. The liquid composition, iron slag composition and slag-based iron removal rate after iron removal are shown in Table 4.
[0024] Table 4 Iron removal results
[0025]
Embodiment 3
[0027] The composition of a low-nickel sulfur nitric acid leaching solution is shown in Table 5.
[0028] Table 5 Low nickel sulfonium nitric acid leaching solution composition (g / L)
[0029]
[0030] Measure 10L of the above solution, heat it to 100~150℃, react for 1~5h, pyrolyze to produce iron slag, NOx and water are recovered and recycled. After the reaction is completed and filtered, the filter residue is hydrolyzed iron slag, and the filtrate is the liquid after iron removal.
[0031] Test results: 973.5g of iron slag and 5100ml of solution were produced. Table 6 shows the liquid composition, iron slag composition and slag-based iron removal rate after iron removal.
[0032] Table 6 Iron removal results
[0033]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com