Vaccine composition, kit and application
A technology of vaccine composition and kit, applied in the direction of vaccines, multivalent vaccines, veterinary vaccines, etc., can solve the problems of CDV virus differences, harm, loss, etc., and achieve good prevention and control effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
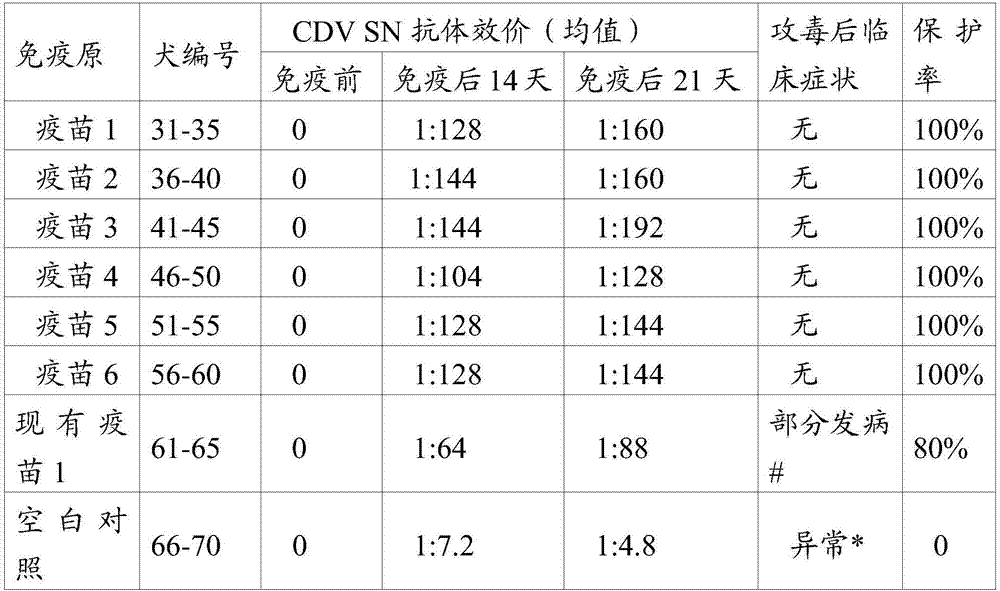
[0026] As an embodiment of the present invention, the content of the inactivated whole virus antigen of the canine distemper virus is 10% before inactivation. 3 -10 9 TCID 50 / ml.
[0027] The term "TCID 50 ” refers to the tissue culture infectious dose, and it is defined as the dilution of virus required to infect 50% of a given batch of inoculated cell cultures. Various methods can be used to calculate the TCID 50, including the Sperman-Karber method, which is used throughout the specification. For a description of the Spearman-Karber method, see BW Mahy, HO Kangro. Virology methods manual, 1996:25-46. In the present invention, when the antigen content is the inactivated antigen content, it means the antigen content before inactivation.
[0028] As a preferred embodiment of the present invention, the content of the inactivated whole virus antigen of the canine distemper virus is 10% before inactivation. 4 -10 6 TCID 50 / ml. As an embodiment of the present invention,...
Embodiment 1
[0062] Example 1 Isolation, identification and content determination of canine distemper virus strain
[0063] 1.1 Isolation and identification of canine distemper virus strains
[0064] Canine distemper virus was isolated from the lung lesions of dogs suspected to have outbreaks of canine distemper by technicians of Pulaike Bioengineering Co., Ltd., and named it CDV C / HN / 001 strain.
[0065] Using CDV H-F: 5'TTAGGGCTCAGGTAGTCCA3', CDV H-R: 5'CTAAG KCCAATTGARATGTGT3' as primers, one-step RT-PCR was performed according to the instructions of the EasyScript One-Step RT-PCR SuperMix (purchased from Beijing Quanshijin Biotechnology Co., Ltd.) Technology to amplify the H gene sequence of CDV C / HN / 001 strain, amplify the target fragment and connect it to the T vector and transform it. Select the positive cloned plasmid and send it to Yingwei Jieji (Shanghai) Trading Co., Ltd. for sequencing. The result is shown in SEQID No.1 , consistent with about 1800bp of the nucleic acid electr...
Embodiment 2
[0069] The preparation of embodiment 2 vaccine composition
[0070] 2.1 Preparation of CDV inactivated whole virus antigen
[0071] The CDV C / HN / 001 strain is inactivated through a final concentration of 0.025% V / V BPL (β-propiolactone), and according to "Chinese Veterinary Pharmacopoeia" (Chinese Veterinary Drug Committee, 2010 edition three, China Agricultural Press , 2010) carried out sterility test, mycoplasma test and exogenous virus test on CDV C / HN / 001, and the results showed that after inactivation of CDV C / HN / 001 strain, it was not polluted by bacteria and mold, nor was it polluted by bacteria or mold Mycoplasma and exogenous virus infection, good purity.
[0072] 2.2 Preparation of CDV subunit antigen
[0073] According to the H protein and F protein gene sequences (see SEQ ID No.1, SEQ ID No.3) of the CDV C / HN / 001 strain measured according to Example 1, and the M protein gene sequence of the CDV SY strain (see NCBI Accession number: KJ466106), respectively design...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com