Preparation method of water electrolysis oxygen evolution non-noble metal catalyst
A non-precious metal and catalyst technology, applied in the electrolysis process, electrolysis components, electrodes, etc., can solve the problems of cumbersome preparation methods, high oxygen evolution overpotential, expensive, etc., and achieve simple preparation methods, many surface defects, and excellent conductivity. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
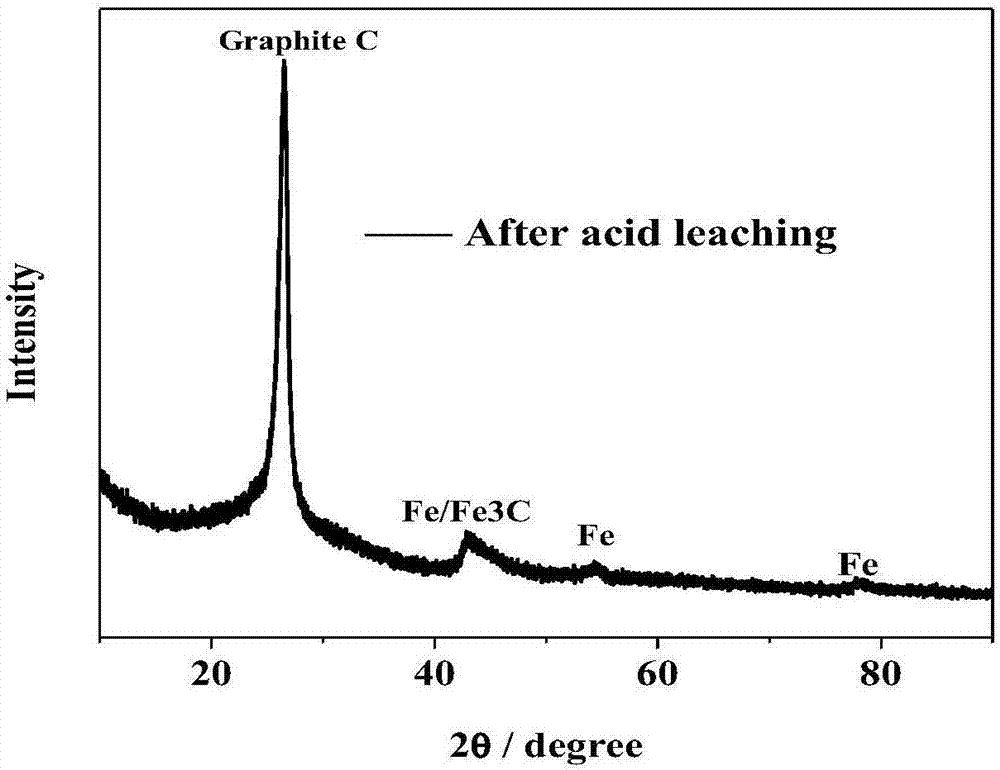
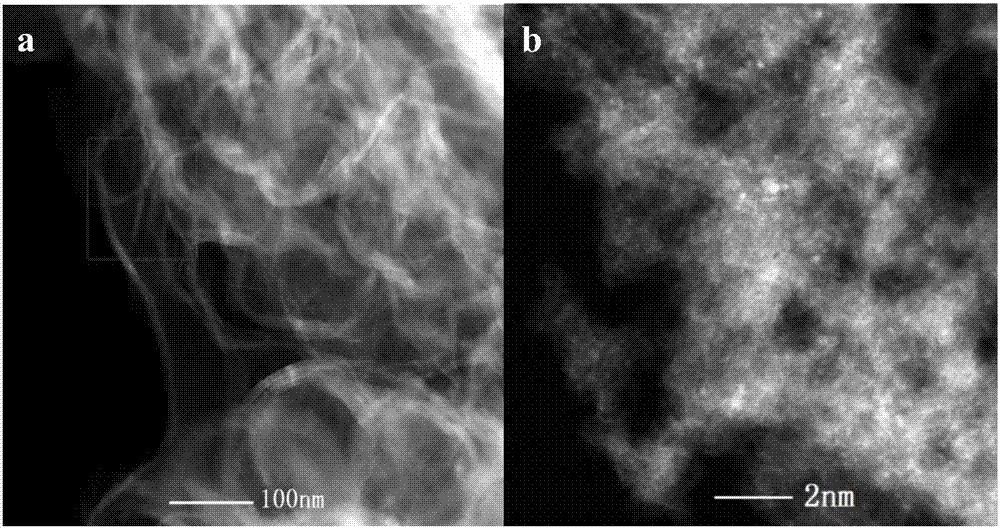
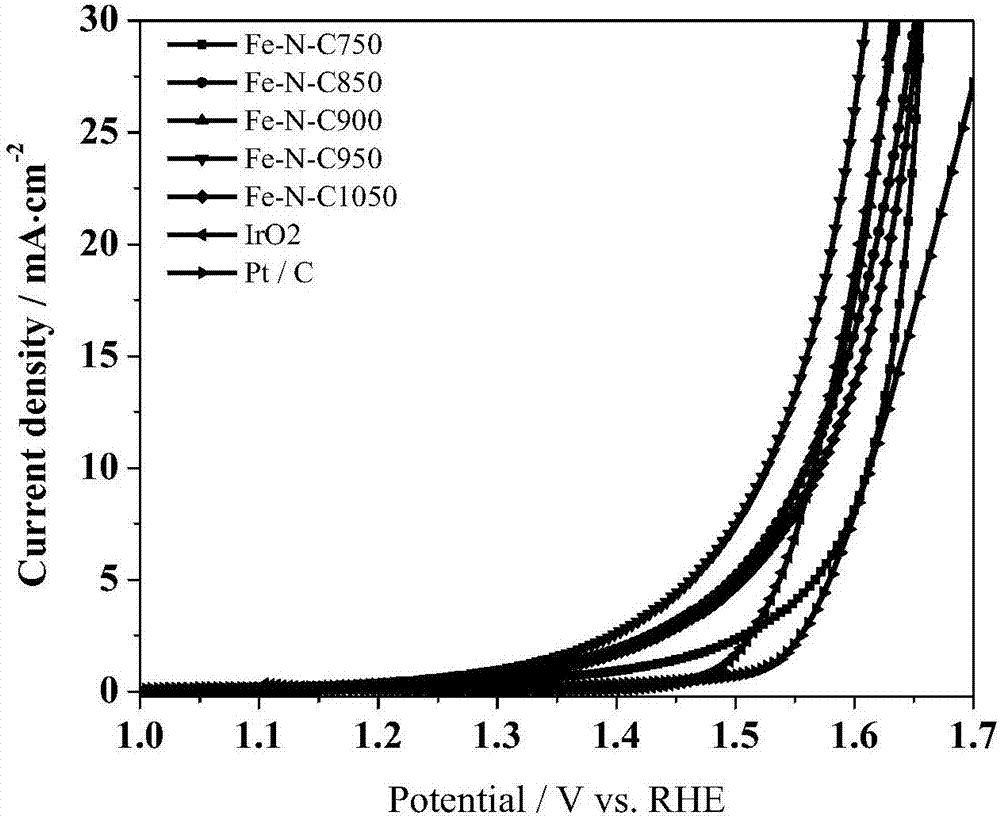
Image
Examples
Embodiment 1
[0033] A method for preparing a non-noble metal catalyst for electrolysis of water, the operation steps are as follows:
[0034] (1) Weigh 1.3g of 2,6-diacetylpyridine into a flat-bottomed flask, add 50ml of absolute ethanol, stir until 2,6-diacetylpyridine is completely dissolved, then add 0.9g of 1,8-diacetylpyridine Aminonaphthalene, stir until 1,8-diaminonaphthalene is completely dissolved, add 0.1g oxalic acid as a catalyst, transfer to an oil bath and raise the temperature to 60°C for synthesis, keep stirring at this temperature for 12 hours, and naturally cool to room temperature;
[0035] (2) Add 2.15 g of ferric chloride hexahydrate while stirring in the solution obtained after cooling in step (1), and stir at room temperature for complexation for 12 hours;
[0036] (3) The solution obtained after the complexation in step (2) is removed by rotary evaporation to remove the solvent, and the remaining solid matter is placed in a blast drying oven and dried at 80° C. for ...
Embodiment 2
[0041] A method for preparing a non-noble metal catalyst for electrolysis of water, the operation steps are as follows:
[0042] (1) Weigh 1.0g of 1,3-diacetylbenzene into a single-necked flask, add 125ml of absolute ethanol, stir until 1,3-diacetylbenzene is completely dissolved, then add 0.9g of 2,6-diacetylbenzene Aminopyridine, stir until 2,6-diaminopyridine is completely dissolved, add 0.08g oxalic acid as a catalyst, transfer to an oil bath and raise the temperature to 25°C for synthesis, keep stirring at this temperature for 24 hours, then cool naturally to room temperature;
[0043] (2) Add 1.29 g of ferric chloride hexahydrate while stirring in the solution obtained after cooling in step (1), and stir at room temperature for complexation for 24 hours;
[0044] (3) The solution obtained after the complexation in step (2) is removed by rotary evaporation to remove the solvent, and the remaining solid matter is placed in a blast drying oven and dried at 90 ° C for 24 hou...
Embodiment 3
[0048] A method for preparing a non-noble metal catalyst for electrolysis of water, the operation steps are as follows:
[0049] (1) Weigh 1.0g of 4,4-diacetylbiphenyl into a single-necked flask, add 200ml of absolute ethanol, stir until 1,3-diacetylbiphenyl is completely dissolved, then add 0.78g of 2,3 -Diaminopyridine, stir until the 2,3-diaminopyridine is completely dissolved, add 0.05g oxalic acid as a catalyst, transfer to an oil bath and raise the temperature to 150°C for synthesis, keep stirring at this temperature for 6 hours, then cool naturally to room temperature ;
[0050] (2) Add 1.12 g of cobalt trichloride hexahydrate while stirring in the solution obtained after cooling in step (1), and stir at room temperature for complexation for 8 hours;
[0051] (3) The solution obtained after the complexation in step (2) is removed by rotary evaporation to remove the solvent, and the remaining solid matter is placed in a blast drying oven and dried at 100 ° C for 8 hours...
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com