Aniline Metal Fullerene Derivatives, Preparation Methods and Controlling Method of Excited State Lifetime
A technology of metallofullerenes and derivatives, applied in organic chemistry, color/spectral characteristic measurement, etc., can solve the problems of unclear control methods, short excited state life, and complicated control methods, and achieve long excited state life and low cost. Inexpensive, highly operable effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] Preparation method of aniline compound with electron-donating group
[0061] 1. the preparation method of the aniline compound of formula (1a), reaction route is:
[0062]
[0063] Specific steps include:
[0064] (1) 1.42mmol (ie 387.66mg) of aldehyde triphenylamine of formula (1c) and 1.56mmol (ie 480mg) of formula (1d) of phosphate were mixed, then dissolved in 30ml of anhydrous tetrahydrofuran, and then 1.7 mmol (i.e. 191.2 mg) of potassium tert-butoxide, stirred and refluxed overnight, then cooled to room temperature, added water, the mixture was extracted with dichloromethane, the extracted product was dried with anhydrous sodium sulfate, the solvent was evaporated, and then filtered on a silica gel column Carry out purification, obtain the product of formula (1b);
[0065] (2) the product of formula (1b) of 1mmol (i.e. 426mg) and the p-formyl phenylboronic acid of 1.2mmol (i.e. 141.6mg) are dissolved in the water of 20ml ethanol and 15ml, then add the lead a...
Embodiment 2
[0083] Metallofullerene Sc 2 C 2 @C 82 -C S preparation method
[0084] ScNi used 2 Alloys were purchased from Beijing Institute of Nonferrous Metals.
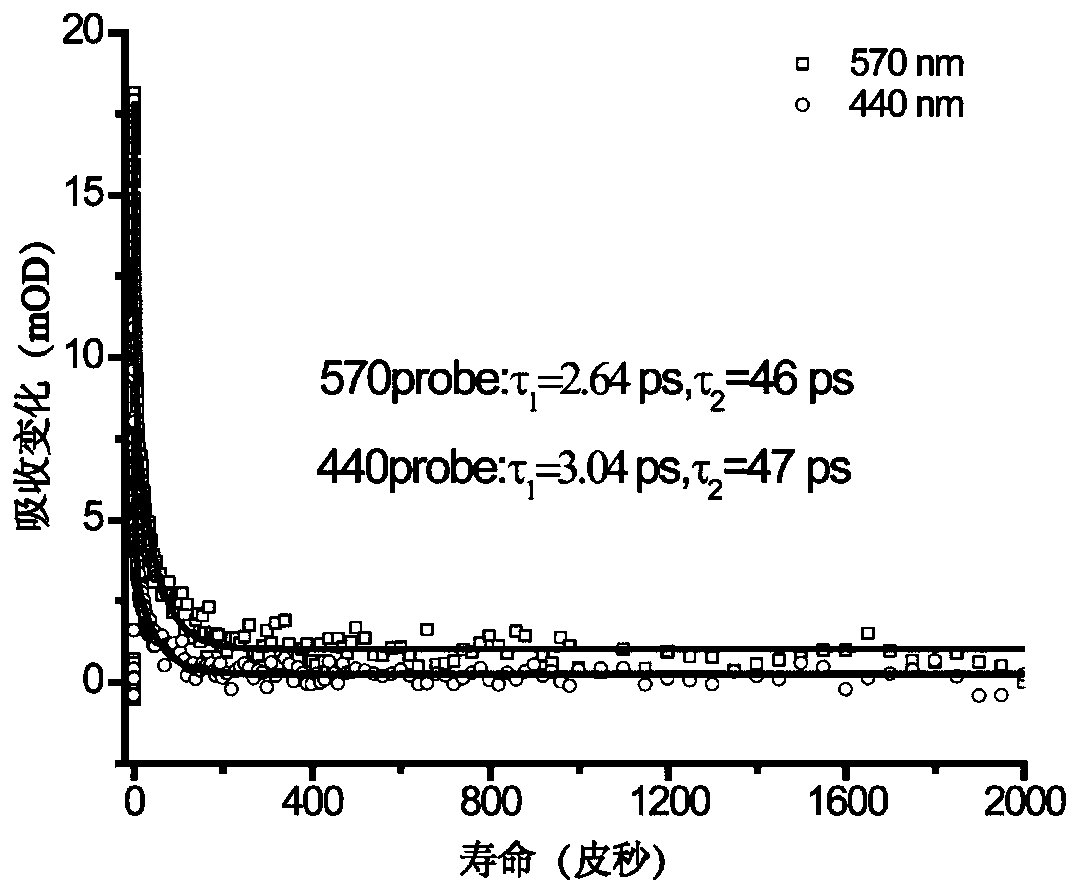
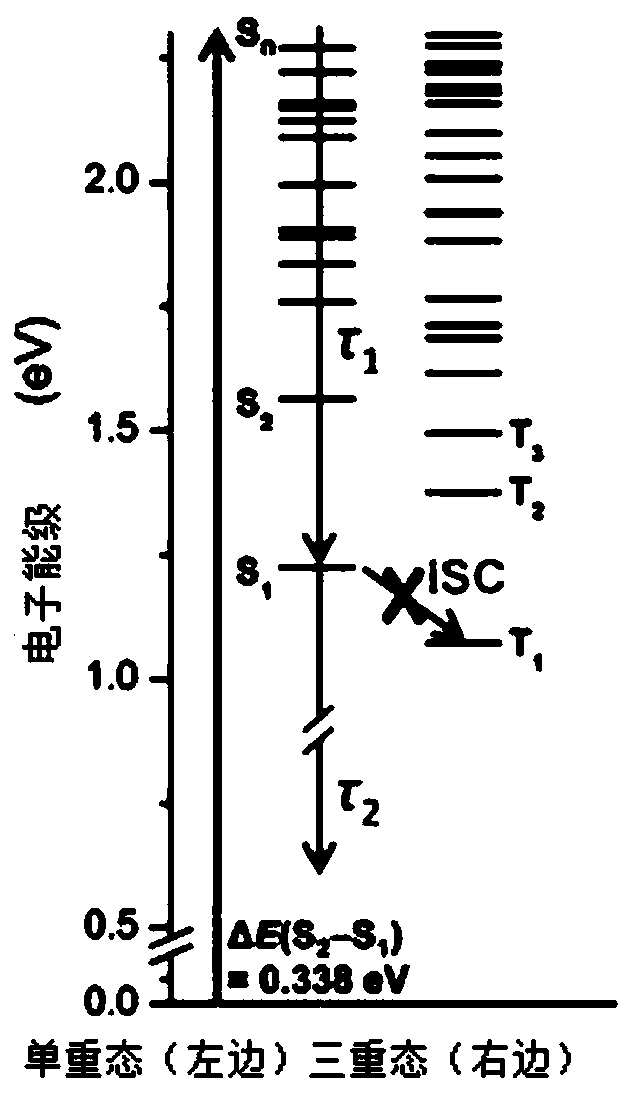
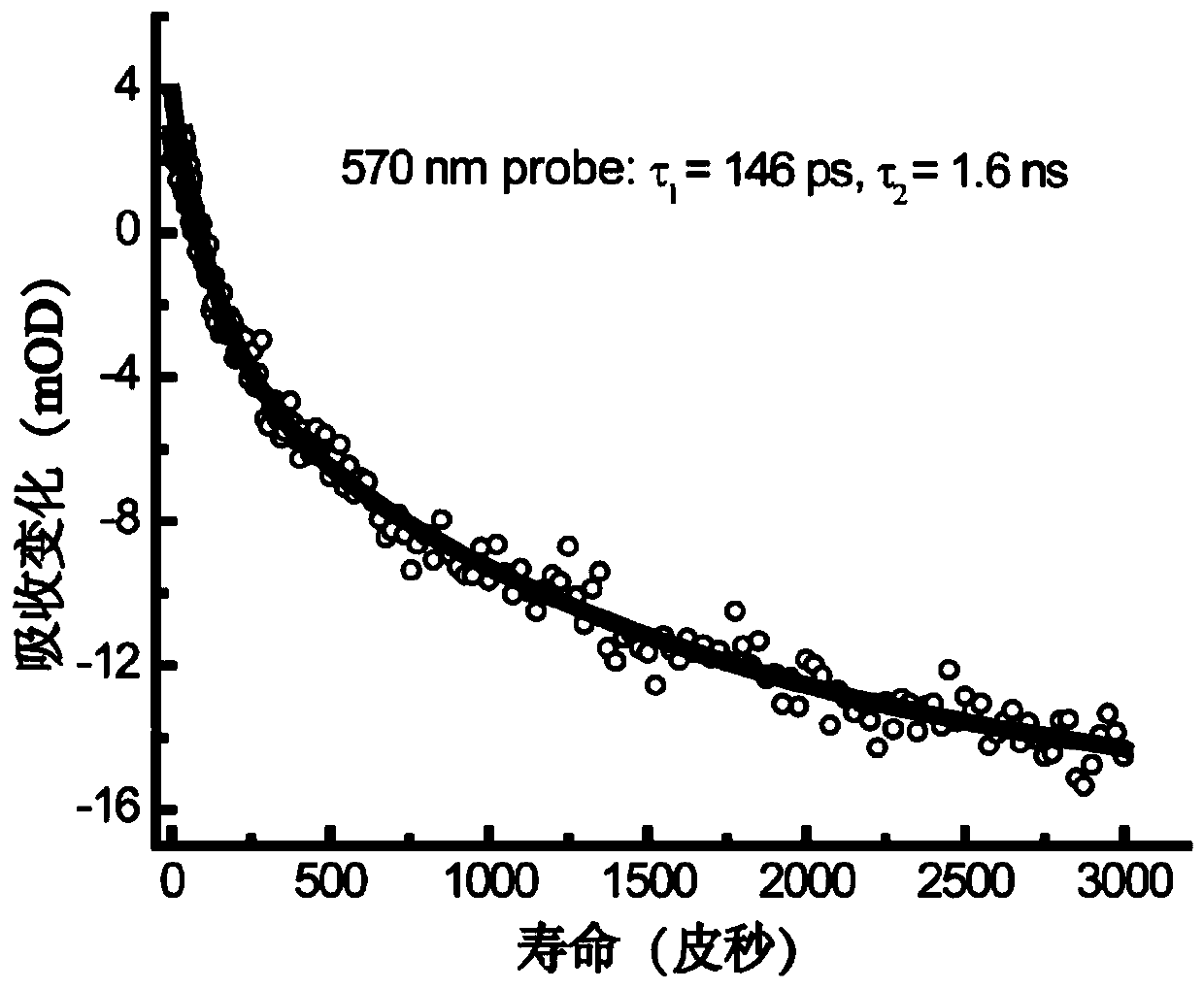
[0085] Among the classes of metallofullerenes, metallofullerenes Sc 2 C 2 @C 82 The yield is relatively high, the properties are excellent, the variety is rich, and the research is relatively mature. Meanwhile, the metallofullerene Sc 2 C 2 @C 82 There are three isomers, namely C S 、C 2V and C 3V configuration, the excited state properties of different isomers are different, for Sc 2 C 2 @C 82 -C S and Sc 2 C 2 @C 82 -C 3V Under the condition of 530nm visible light excitation, the electronic dynamics curves of the excited state are all double-exponential relaxation processes. After analysis, there is no intersystem crossing to the triplet state, and Sc 2 C 2 @C 82 -C 2V Then there is a long-lived triplet relaxation process. Therefore, this application chooses metal fullerene Sc 2 C 2 @C 82 -C SAs ...
Embodiment 3
[0092] Preparation method of aniline metal fullerene derivatives
[0093] 1. the preparation method of the aniline metallofullerene of formula (1), the steps comprise:
[0094]
[0095] (1) Take 0.91×10 3 nmol (ie 1 mg) of Sc 2 C 2 @C 82 -C S The solid sample was dissolved in toluene, and then 0.97×10 3 nmol (ie 0.1mg) of N-ethylglycine and 1.35×10 3 nmol (ie 0.6 mg) of compound 1a was filled with argon as a protective gas, and subjected to cycloaddition reaction, and reacted at 120°C for 20 minutes under stirring conditions;
[0096] (2) After the reactant was cooled for half an hour, the solution was evaporated to dryness, the product was dissolved in toluene, and then separated and purified by high performance liquid chromatography. The separation and purification steps were: use Buckyprep column (20 × 250mm, Cosmosil) to separate, In the experiment, toluene was used as the mobile phase, the flow rate of toluene was 12ml / min, the sample concentration was 1mg / ml, 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com